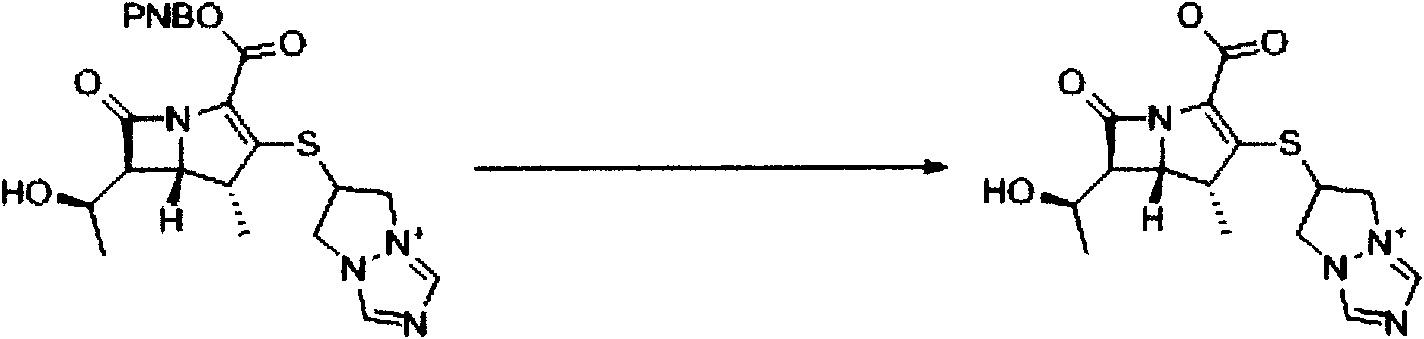Method for preparing biapenem by using micro-reaction technology
A biapenem and micro-reaction technology, which is applied in the field of preparing biapenem by using micro-reaction technology, can solve the problems of many by-products, low yield, long reaction time, etc., to prevent side reactions, increase specific surface area, The effect of reducing the amount of solvent used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
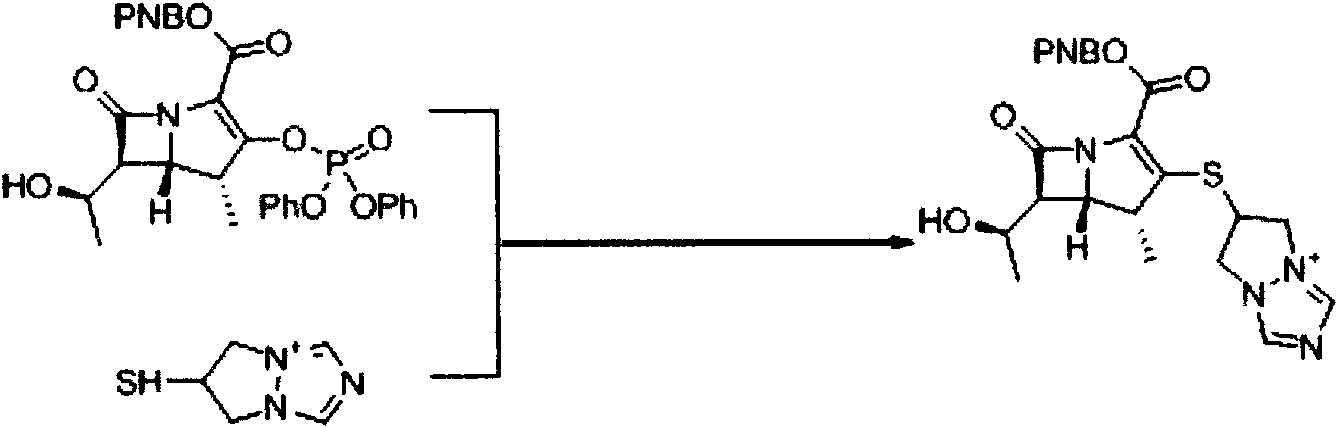
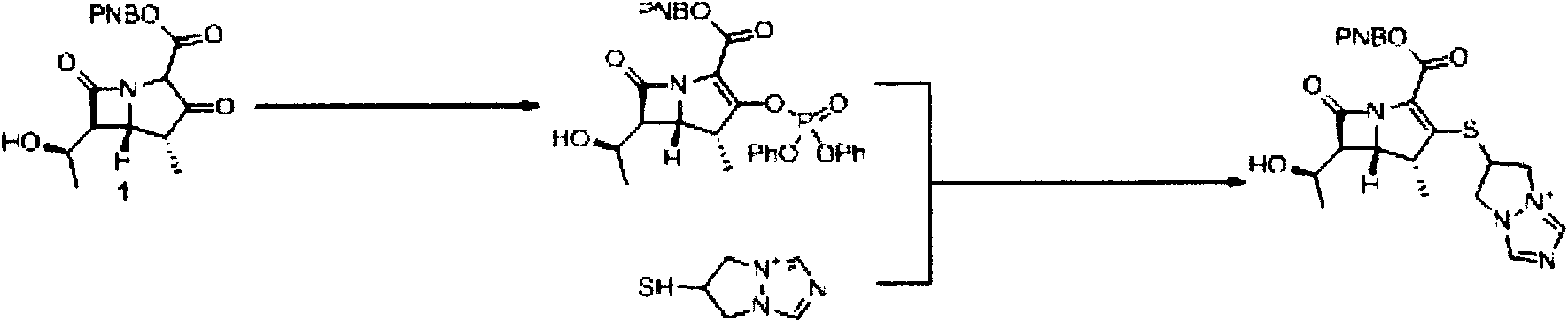
[0043] Synthesis of (4R, 5S, 6S)-3-[(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazolium-6-yl by microreaction technique )]-thio-6-[(R)-1-hydroxyethyl]-4-methylcarbapenicill-2-ene-2-p-nitrophenyl ester (1)
[0044] This reaction was carried out using a microchannel reaction system (FRX 100, Syrris). The first reserve part of the reaction system is loaded with 4.88g (8.0mmol) (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)-1-hydroxyethyl]-4-methyl carbon Penicillium-2-ene-2-p-nitrophenyl ester (2) and 30ml of anhydrous acetonitrile and slightly heated compound (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)- 1-Hydroxyethyl]-4-methylcarbapillin-2-en-2-p-nitrophenyl ester (2) was dissolved. The second storage portion of the reaction system was charged with 1.14 g (8.0 mmol) of 6-mercapto-(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazole ylide ( 3), 1.2ml (8.8mmol) diisopropylethylamine and 30ml anhydrous acetonitrile make it dissolve.Microchannel mixing part is the volume of reaction part is 1...
Embodiment 2
[0046] Synthesis of (4R, 5S, 6S)-3-[(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazolium-6-yl by microreaction technique )]-thio-6-[(R)-1-hydroxyethyl]-4-methylcarbapenicill-2-ene-2-p-nitrophenyl ester (1)
[0047] This reaction was carried out using a microchannel reaction system (FRX 100, Syrris). The first reserve part of the reaction system is loaded with 4.88g (8.0mmol) (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)-1-hydroxyethyl]-4-methyl carbon Penicillium-2-ene-2-p-nitrophenyl ester (2) and 30ml of anhydrous acetonitrile and slightly heated compound (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)- 1-Hydroxyethyl]-4-methylcarbapillin-2-en-2-p-nitrophenyl ester (2) was dissolved. The second storage portion of the reaction system was charged with 1.14 g (8.0 mmol) of 6-mercapto-(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazole ylide ( 3), 1.2ml (8.8mmol) diisopropylethylamine and 30ml anhydrous acetonitrile make it dissolve.Microchannel mixing part is the volume of reaction part is 1...
Embodiment 3
[0049] Synthesis of (4R, 5S, 6S)-3-[(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazolium-6-yl by microreaction technique )]-thio-6-[(R)-1-hydroxyethyl]-4-methylcarbapenicill-2-ene-2-p-nitrophenyl ester (1)
[0050] This reaction was carried out using a microchannel reaction system (FRX 100, Syrris). The first reserve part of the reaction system is loaded with 4.88g (8.0mmol) (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)-1-hydroxyethyl]-4-methyl carbon Penicillium-2-ene-2-p-nitrophenyl ester (2) and 30ml of anhydrous acetonitrile and slightly heated compound (4R, 5S, 6S)-3-diphenylphosphate oxy-6-[(R)- 1-Hydroxyethyl]-4-methylcarbapillin-2-en-2-p-nitrophenyl ester (2) was dissolved. The second storage portion of the reaction system was charged with 1.14 g (8.0 mmol) of 6-mercapto-(6,7-dihydro-5H-pyrazol[1,2-a][1,2,4]triazole ylide ( 3), 1.2ml (8.8mmol) diisopropylethylamine and 30ml anhydrous acetonitrile make it dissolve.Microchannel mixing part is the volume of reaction part is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com