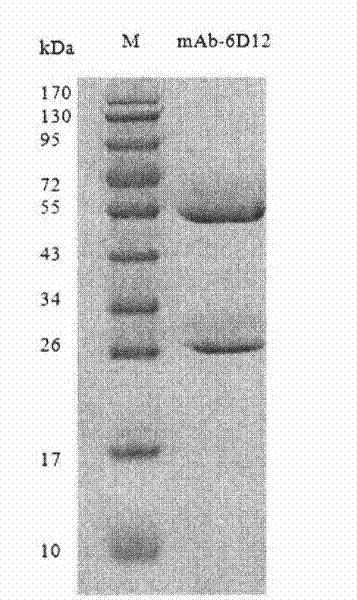
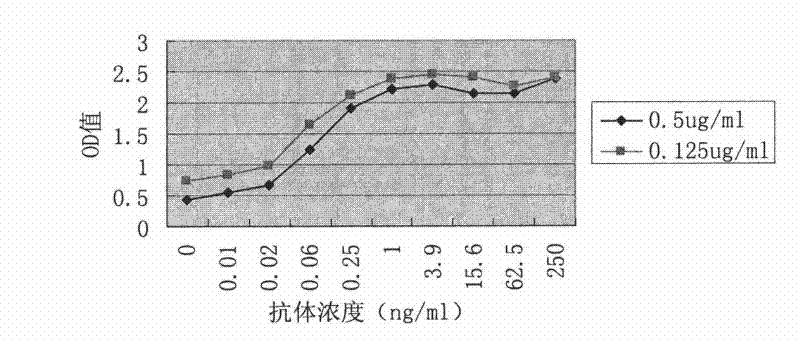
Tetanus toxoid monoclonal antibody and preparation method and application thereof
A monoclonal antibody and anti-tetanus technology, applied in the field of biological immunology, can solve the problems of limited application of tetanus human blood immunoglobulin, high price, and difficult source of human blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, preparation of anti-tetanus toxoid mouse monoclonal antibody
[0076] 1. Preparation of anti-tetanus toxoid monoclonal antibody hybridoma cell line
[0077] 1. Immunogen
[0078] Tetanus toxoid was purchased from the Production Department of Shanghai Institute of Biological Products.
[0079] 2. Immunization of Balb / c mice
[0080] Balb / c mice were purchased from Shanghai Slack Co., Ltd. All Balb / c mice used for immunization were 3-week-old, female, standardized disease-free, healthy purebred mice, which met the US FDA standards.
[0081] 3. Balb / c mouse immunization method
[0082] The first immunization: Mix 50 μg (250 μl) of antigen with 250 μl of aluminum adjuvant, prepare a 500 μl solution, and inject it into Balb / c mice at multiple points and paws subcutaneously. The second immunization: three weeks after the first immunization, the second immunization is carried out in the same way and with the same dose. The third and fourth immunizations: t...
Embodiment 2
[0098] Example 2, Cloning and Identification of Anti-tetanus Toxoid Monoclonal Antibody Variable Region Coding Sequence
[0099] Using the cDNA of the anti-tetanus toxoid hybridoma cell line (6D12) screened in Example 1 as a template, PCR technology was used to clone the anti-tetanus toxoid mouse monoclonal antibody variable region gene; after sequencing, no mutation and no termination were selected. The sequence of the codon, using 5'RACE technology to clone the functional V L and V H Gene.
[0100] 1. Cloning of the variable region coding sequence of anti-tetanus toxoid murine monoclonal antibody
[0101] (1) Extracting total RNA of anti-tetanus toxoid monoclonal antibody from hybridoma cell lines
[0102] Extracted with FAST1000 kit of Shanghai Feijie Biological Company.
[0103] (1) Take 4×10 5 Hybridoma cells, 1000rpm×3min, discard the supernatant. Wash once with PBS. Resuspend the cells in 100 μl PBS and place in a centrifuge tube.
[0104] (2) Add 1ml of RB1 s...
Embodiment 3
[0173] Example 3, Construction of anti-tetanus toxoid human-mouse chimeric monoclonal antibody eukaryotic expression vector
[0174] light chain V L gene and human Ig C L The genes were spliced to form a light chain chimeric gene; a BamHI restriction endonuclease site was introduced at the 5' end of the light chain, and an EcoRI restriction endonuclease site was introduced at the 3' end of the light chain. Similarly, construct the heavy chain chimeric gene. The above light chain / heavy chain genes were respectively inserted into the monoclonal restriction site of pcDNA3.1(+ / -) expression vector (purchased from Invitrogen) to construct the expression vector of anti-tetanus toxoid human-mouse chimeric antibody.
[0175] (1) Design the upstream and downstream products according to the conventional method, and introduce BamHI single enzyme cutting site at the 5' end of the heavy chain / light chain variable region gene by PCR technology, and introduce EcoR at the 3' end of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com