Method for separating zirconium and hafnium
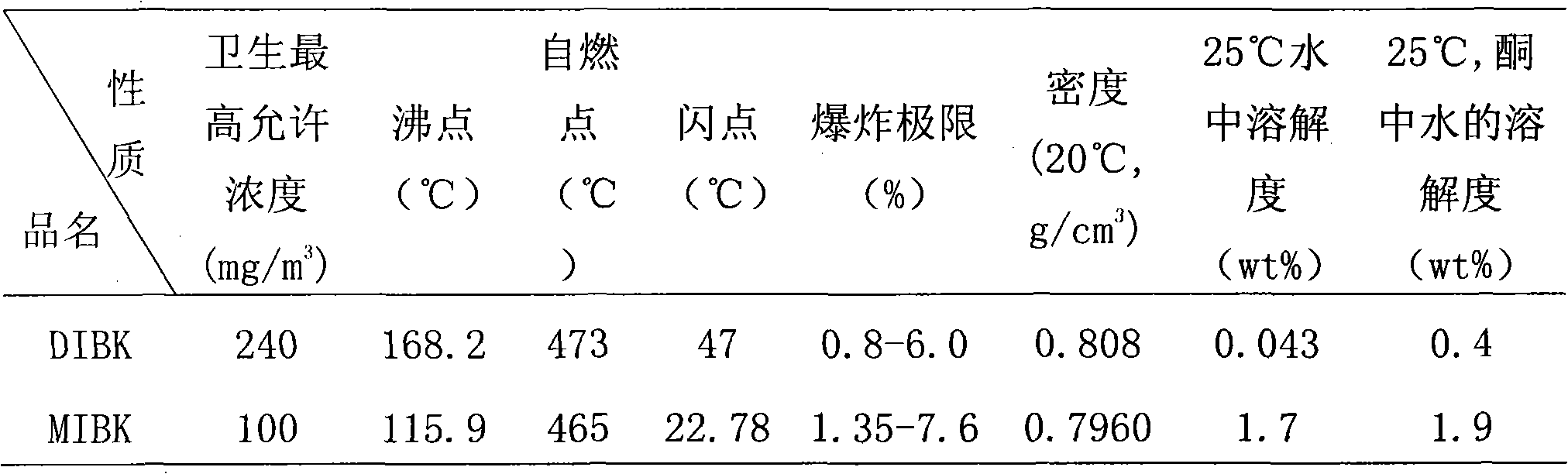
A technology of zirconium hydroxide and hafnium hydroxide, which is applied in the field of separating zirconium and hafnium, can solve the problems of high water solubility of MIBK, easy pollution of the environment, and easy occurrence of fires, and solve the problems of large solvent loss, low mass transfer, boiling flash and The effect of high flash point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for separating zirconium and hafnium, the specific steps are as follows:
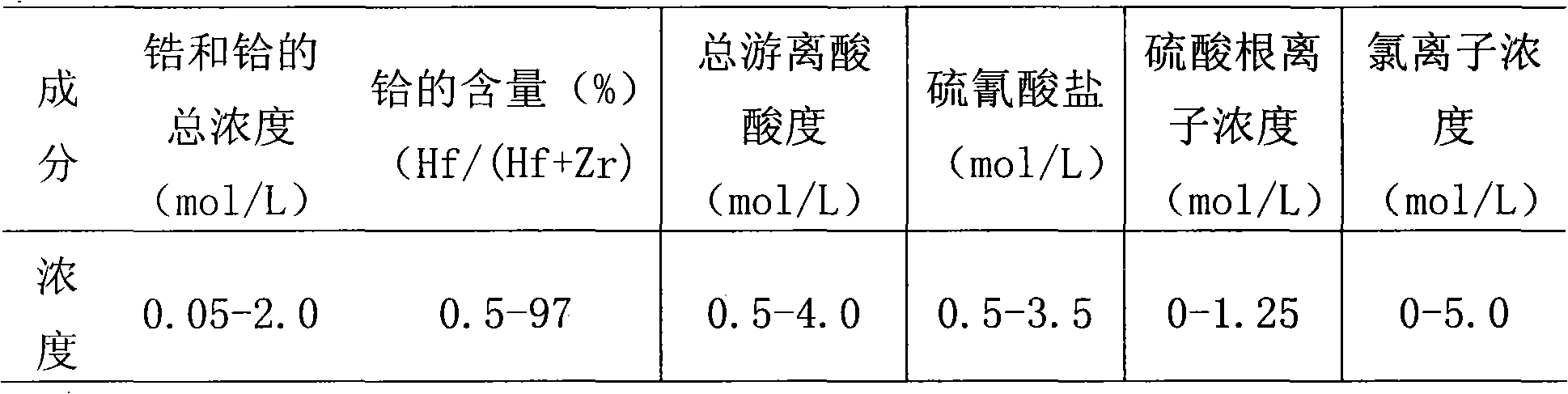
[0040] (1) Preparation of acidic feed liquid: the composition of the water phase is the initial total concentration of zirconium and hafnium ions of 1.3 mol / L, of which the concentration of hafnium ions is about 0.016 mol / L, and the acidity of the water phase is 1.0 mol / L, [NH 4 SCN]=3.0mol / L, without adding (NH 4 ) 2 SO 4 .
[0041] (2) Using DIBK as the extractant, first pre-extract the extractant with an equal volume of 3.0 mol / L thiocyanic acid once, then use the extractant as the organic phase and the feed liquid as the water phase to control the comparison (organic phase: water Phase) is 2:1, the feed solution is subjected to single-stage extraction at room temperature, and the two-phase mixing time is 15 minutes. The zirconium remains in the water phase to obtain a hafnium-free zirconium solution, which is then precipitated with ammonia to obtain zirconium hydroxide precipitation , And...
Embodiment 2
[0046] The organic phase is composed of 10% (v / v) TBP and 90% (v / v) DIBK and pre-extracted once with an equal volume of 1.0 mol / L HSCN. The aqueous phase is composed of an initial total concentration of zirconium and hafnium ions of 1.5 mol / L, the concentration of hafnium ion is 0.018mol / L, the acidity of the water phase is 1.0mol / L, [NH 4 SCN]=3.0mol / L, (NH 4 ) 2 SO 4 The addition amount is 0.8mol / L, the control ratio is 2:1, single-stage extraction is performed at room temperature, the two-phase mixing time is 5 minutes, and then precipitation with ammonia water is used to obtain zirconium hydroxide precipitation and a load organic phase containing hafnium; The loaded organic phase was washed with 2.0 mol / L hydrochloric acid and 12 mol / L sulfuric acid for back extraction. The ratio of washing and back extraction was 1:2, and the mixing time of the two phases was 5 minutes to obtain 96% (Hf / (Hf +Zr)) hafnium-rich solution, using ammonia water for precipitation to obtain hafniu...
Embodiment 3
[0049] The organic phase is composed of 10% (v / v) TRPO and 90% (v / v) DIBK and pre-extracted once with an equal volume of 2.0 mol / L HSCN. The aqueous phase is composed of an initial total concentration of zirconium and hafnium ions of 2 mol / L , The concentration of hafnium ion is 0.02mol / L, the acidity of the water phase is 0.5mol / L, [NH 4 SCN]=3.0mol / L, without adding (NH 4 ) 2 SO 4 , The control ratio is 2:1, single-stage extraction is carried out at room temperature, the two-phase mixing time is 10 minutes, and then precipitation with ammonia water to obtain zirconium hydroxide precipitate and hafnium-containing supported organic phase; use 0.5mol of the supported organic phase / L hydrochloric acid for washing and 6.0mol / L sulfuric acid for back extraction, the ratio of washing and back extraction is 1:2, the two-phase mixing time is 10 minutes, and 90% (Hf / (Hf+Zr)) enrichment is obtained. The hafnium solution is precipitated with ammonia water to obtain hafnium hydroxide prec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com