Method for electrolytically preparing hydrogen from formic acid
A formic acid, hydrogen technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
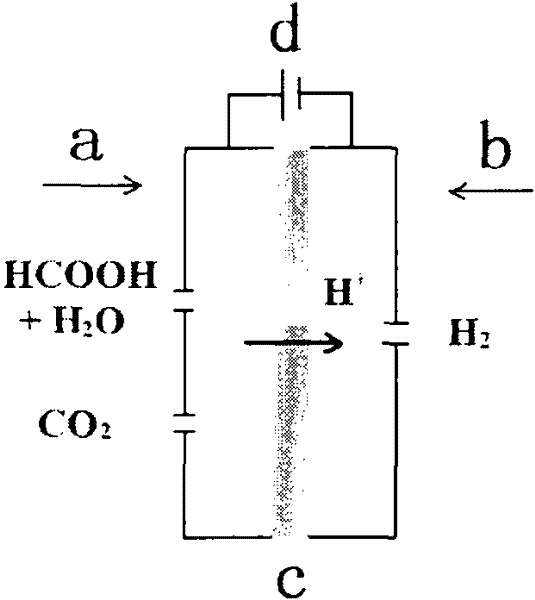
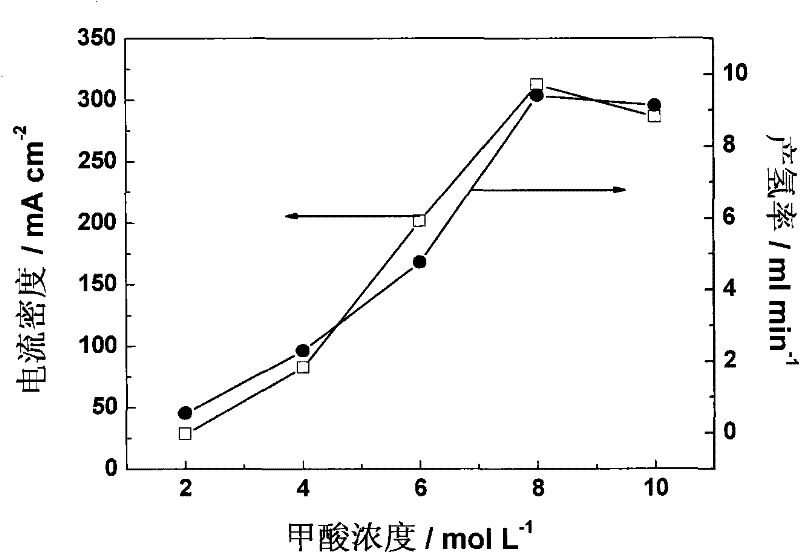
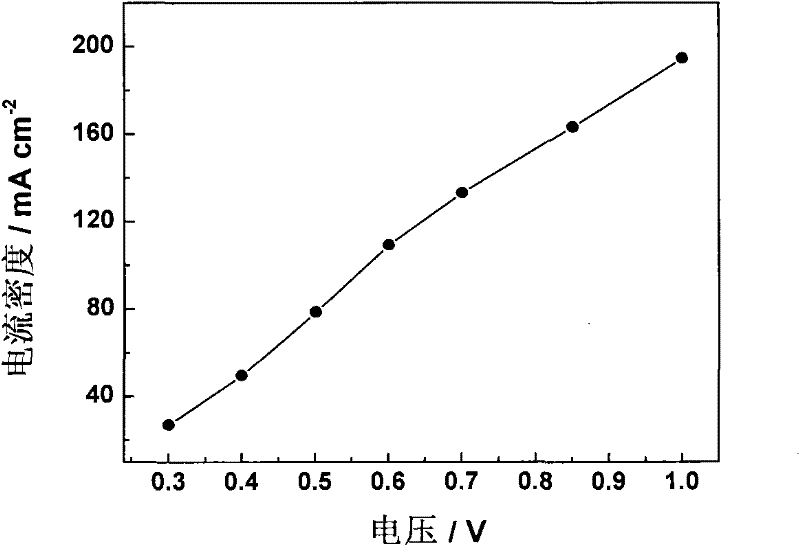
[0028] according to figure 1 Assemble a polymer electrolyte membrane electrolyzer, the cathode and anode diffusion layers are made of carbon paper (Toray-060) with PTFE hydrophobic treatment, wherein the anode PTFE mass percentage is 15%, and the cathode PTFE mass percentage is 30%. The anode catalyst is Pd / C, which is sprayed on the anode diffusion layer, and the loading capacity is 2mg / cm 2 ; The cathode catalyst is Pt / C, which is sprayed on the cathode diffusion layer with a loading of 1 mg / cm 2 . The electrolyte membrane is -115 membrane, electrode effective area is 4cm 2 . 2-10M formic acid solution is passed into the anode of the electrolyzer at a flow rate of 1ml / min, and nitrogen gas is passed into the cathode at a flow rate of 20 sccm. Use a PAR273A electrochemical workstation (potentiostat / galvanostat, Princeton Applied Research, USA) as a DC power supply, apply a voltage of 0.3-1V across the electrolytic cell, and control the temperature of the electrolytic ce...
Embodiment 2
[0032] Figure 4 For the use of formic acid aqueous solution as fuel, the electrolytic production of hydrogen by polymer electrolyte membrane electrolyzer is combined with proton exchange membrane fuel cell (PEMFC).
[0033] In the figure, the electrolysis power supply is a No. 5 battery (rated voltage 1.5V); the electrolyzer is two electrolysis electrodes connected in series, and constituted together with bipolar plates. Due to the existence of ohmic loss, the measured voltages of the two series electrolytic electrodes are all in the range of 0.65-0.68V.
[0034] The anode catalyst of the electrolysis electrode is Pd / C, and the loading capacity is 3mg / cm 2 , the cathode catalyst is Pt / C, and the loading capacity is 1mg / cm 2 , the total electrode area is 16cm 2 .
[0035] In the figure, the cathode and anode catalysts of the proton exchange membrane fuel cell (PEMFC) are both Pt / C, and the loading capacity is 0.4mg / cm 2 , and the cathode adopts air self-breathing mode, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com