Aspidinol compound and application thereof in preparing medicines for resisting drug-resistant bacteria
A compound, the technology of cotton horse phenol, applied in the field of pharmacy, can solve problems such as failure and reduction of drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
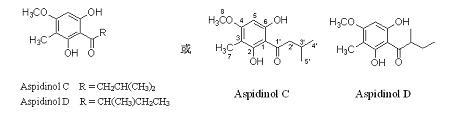
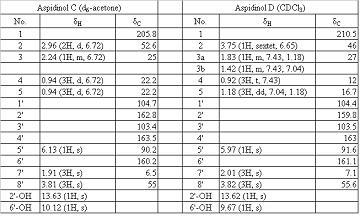
[0029] Take 2 kg of dried hypericum (Hypericum chinense L.) fruit leaves, crush them, soak and extract with 95% ethanol (3 L × 3 times), combine the extracts and evaporate to dryness under reduced pressure to obtain 437 g of extract. The extract was eluted with 200-300 mesh silica gel (6×60 cm silica gel column), petroleum ether-ethyl acetate (100:0 ~ 0:100) gradient, and detected by silica gel thin-layer chromatography. The fractions eluted with petroleum ether-ethyl acetate 70:30 were combined and evaporated to dryness to obtain an orange-yellow waxy solid, which was re-chromatographed on a silica gel column with petroleum ether-acetone (80:20) as the eluent to obtain yellow crystals. Continue to purify by silica gel column chromatography, the eluent is petroleum ether-acetone (90:10), to obtain 10 g of yellow crystals. It was detected as a pure compound by forward and reverse phase silica gel thin-layer chromatography and HPLC, and was identified as aspidinol C by spectral ...
Embodiment 2
[0031] Embodiment 2 antibacterial experiment
[0032] Before the minimum inhibitory concentration (MIC) test, all test strains were inoculated on agar medium (Oxoid) and incubated at 37oC for 24 h. The control antibacterial drug norfloxacin and the test compound samples aspidinol C and aspidinol D solutions were added to the corresponding wells at a certain solubility. The matrix was Cation adjusted Mueller-Hinton broth (CAMHB). Incubate at 37oC for 18 h. For MIC determination, 20 μL of 5 mg / mL bromodimethylthiazolediphenyltetrazolium blue (MTT; Sigma) staining solution was added to each well and incubated for 20 min. Bacterial growth is judged by a color change from yellow to dark blue. Based on observations, the lowest concentration that results in no growth of bacteria is the MIC value.
[0033] The results show that the minimum inhibitory concentration MIC of fluoroquinolones norfloxacin is 32 μg / mL (100 μM), and the MICs of compounds aspidinol C and aspidinol D of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com