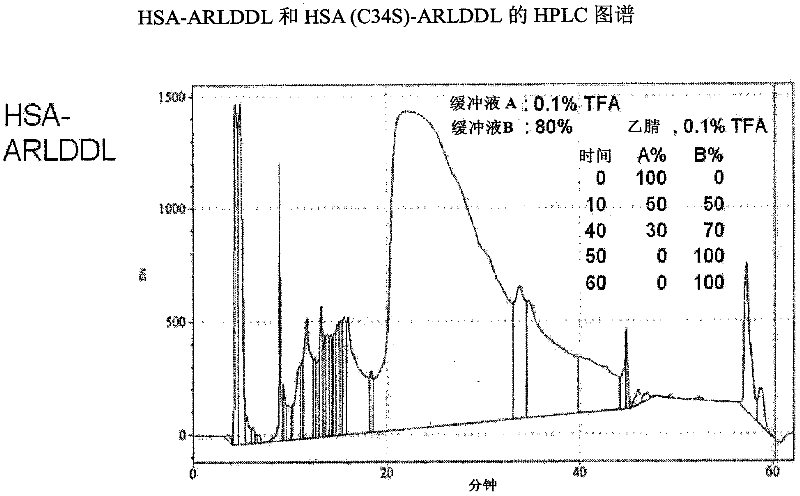
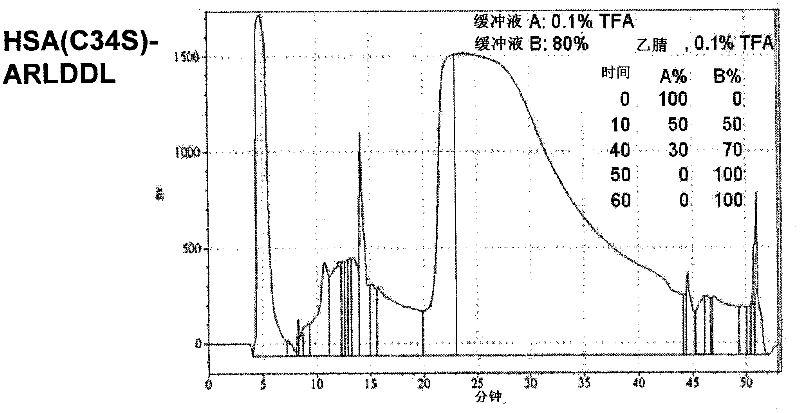
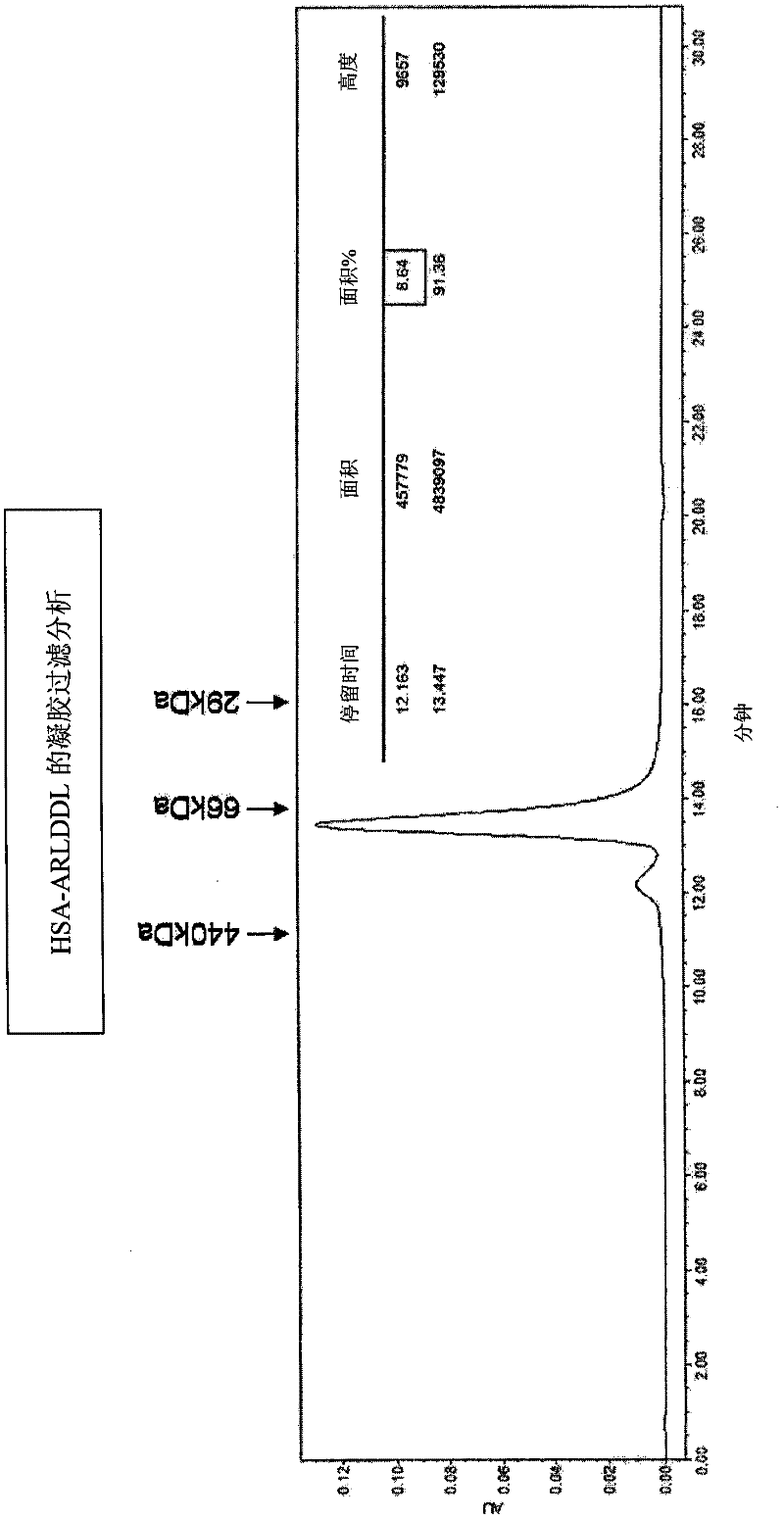
Polypeptides selective for av ss3 integrin conjugated with a variant of human serum albumin (HSA) and pharmaceutical uses thereof
A technology of human serum albumin and integrin, applied in the field of treatment and prevention of avβ3 integrin-related diseases, can solve the problem of reducing polypeptide activity and achieve high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Construction of the gene encoding HSA(C34S)-ARLDDL
Embodiment 1A
[0185] Construction of a gene encoding HSA(C34S)-ARLDDL by overlap extension PCR and ligation reaction
[0186] Take HSA (invitrogen clone ID: IOH23065) was used as a template to construct the HSA C34S gene. The C34S mutation was generated by two-step polymerase chain reaction (PCR). The first step of PCR was performed using sense primers containing C34S mutation sites and antisense primers containing KpnI, SacII restriction enzyme sites and TAA terminators. A second-step PCR was performed using a sense primer containing a BstI restriction site and a secretion signal peptide, and an antisense primer containing a KpnI, SacII restriction site and a TAA terminator. The secretion signal sequence of HSA propeptide, alpha factor propeptide derived from Saccharomyces cerevisiae, or a fusion protein of HSA propeptide and alpha factor propeptide can be used for the expression of secreted protein. Structural gene ARLDDL was amplified by PCR using a sense primer containing a KpnI r...
Embodiment 1B
[0188] Construction of a gene encoding HSA(C34S)-ARLDDL by gene synthesis
[0189] DNA encoding the secretion signal peptide sequence HSA(C34S)-ARLDDL was synthesized. The secretion signal sequence of HSA propeptide, alpha factor propeptide derived from Saccharomyces cerevisiae, or a fusion protein of HSA propeptide and alpha factor propeptide can be used for the expression of secreted protein. The resulting gene product was cloned into a yeast recombinant vector with appropriate restriction sites. Then the recombinant vector was transformed into Escherichia coli XL1-blue strain. Colonies were selected on agar plates containing low-salt LB (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 1.5% agar, pH 7.0) and 25 μg / ml antibiotic Zocine. The Escherichia coli XL1-blue clone was selected, and the plasmid was isolated and sequenced.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com