Method for improving chemical stability of proton exchange membrane
A proton exchange membrane, chemical stability technology, applied in electrical components, battery electrodes, circuits, etc., can solve many problems and achieve the effect of reducing membrane degradation, simple method and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
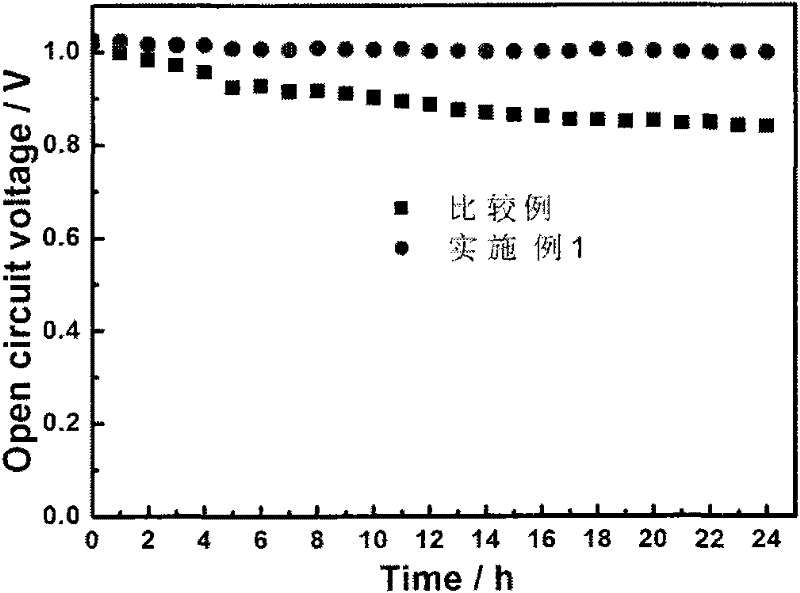
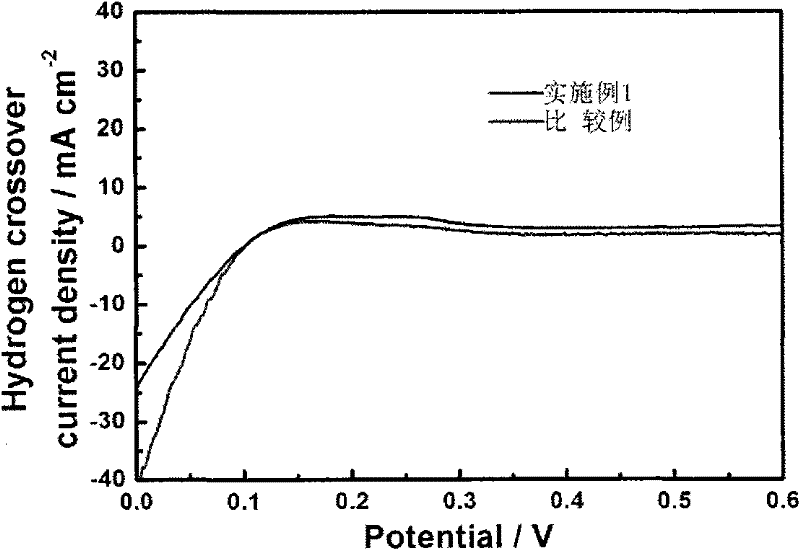
[0018] Prepare 1L 0.05mmol / L Zr(NO3)4 solution, immerse 10g perfluorosulfonic acid membrane (Nafion212) in the solution, take out the membrane after 24h to obtain a trace amount of Zr 4+ Typed Nafion212 (referred to as Zr 4+ -Nafion212), Zr 4+ The number of moles is 20% of the number of moles of sulfonic acid groups in the perfluorosulfonic acid proton exchange membrane. The membrane-electrode three-in-one assembly was hot-pressed at 140°C, activated after the battery was assembled, and then the chemical stability of the membrane was investigated in the open-circuit accelerated test. The operating conditions were: the battery temperature was 80°C, the humidification temperature was 64°C, and the relative humidity Control at 50%, the hydrogen and oxygen intake flow rate is 40ml / min, the intake pressure is 0.2MPa, and it runs continuously for 24 hours.
[0019] The experimental results show that: Zr 4+ -The OCV rate of Nafion212 is significantly lower than that of the compara...
Embodiment 2
[0021] Prepare 1L 0.05mmol / L Mg(NO 3 ) 2 solution, immerse 10g perfluorosulfonic acid membrane (Nafion212) in the solution, take out the membrane after 24h to obtain a trace amount of Mg 2+ Typed Nafion212 (referred to as Mg 2+ -Nafion212), Mg 2+ The number of moles is 10% of the number of moles of sulfonic acid groups in the perfluorosulfonic acid proton exchange membrane. The membrane-electrode three-in-one assembly was hot-pressed at 140°C, activated after the battery was assembled, and then the chemical stability of the membrane was investigated in the open-circuit accelerated test. The operating conditions were: the battery temperature was 80°C, the humidification temperature was 64°C, and the relative humidity Control at 50%, the hydrogen and oxygen intake flow rate is 40ml / min, the intake pressure is 0.2MPa, and it runs continuously for 24h.
Embodiment 3
[0023] Prepare 1L 0.05mmol / L Ca(NO 3 ) 2 Solution, immerse 10g perfluorosulfonic acid membrane (Nafion212) in the solution, take out the membrane after 24h to obtain trace Ca 2+ Typed Nafion212 (referred to as Ca 2+ -Nafion212), Ca 2+ The number of moles is 10% of the number of moles of sulfonic acid groups in the perfluorosulfonic acid proton exchange membrane. The membrane-electrode three-in-one assembly was hot-pressed at 140°C, activated after the battery was assembled, and then the chemical stability of the membrane was investigated in the open-circuit accelerated test. The operating conditions were: the battery temperature was 80°C, the humidification temperature was 64°C, and the relative humidity Control at 50%, the hydrogen and oxygen intake flow rate is 40ml / min, the intake pressure is 0.2MPa, and it runs continuously for 24h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com