Marker for predicting therapeutic effect on hepatitis c, method for predicting therapeutic effect on hepatitis c, and prophylaxis or therapeutic agent for hepatitis c
A therapeutic effect and marker technology, applied in the fields of markers for predicting the therapeutic effect of hepatitis C, predicting the therapeutic effect of hepatitis C, and preventive or therapeutic agents for hepatitis C, can solve the problem of correct treatment effect. Problems such as prediction and inability to transfer SNP to achieve the effect of easy judgment and clear risk value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0162] The preparation of the oligo(or poly)nucleotide can be carried out by a known method, but when the oligo(or poly)nucleotide is DNA, it can be obtained, for example, from blood cells such as lymphocytes or granulocytes in blood as a sample, by Salting out, PCI (phenol chloroform extraction) method, a method using a commercially available DNA extraction kit, etc., or other known methods are performed.
[0163] In addition, when the oligo (or poly) nucleotide is mRNA, the method of making the solution acidic to extract from the water layer in the PCI method, the method of using an Oligo-dT column, etc., or using a commercially available RNA extraction kit can be used. method, other known methods.
[0164] "Nucleic Acid Amplification"
[0165] When the amount of nucleic acid obtained from a sample is small, it can be amplified by a known method if necessary.
[0166] When the nucleic acid is DNA, PCR method or the like can be used.
[0167] When the nucleic acid is mRNA,...
Embodiment
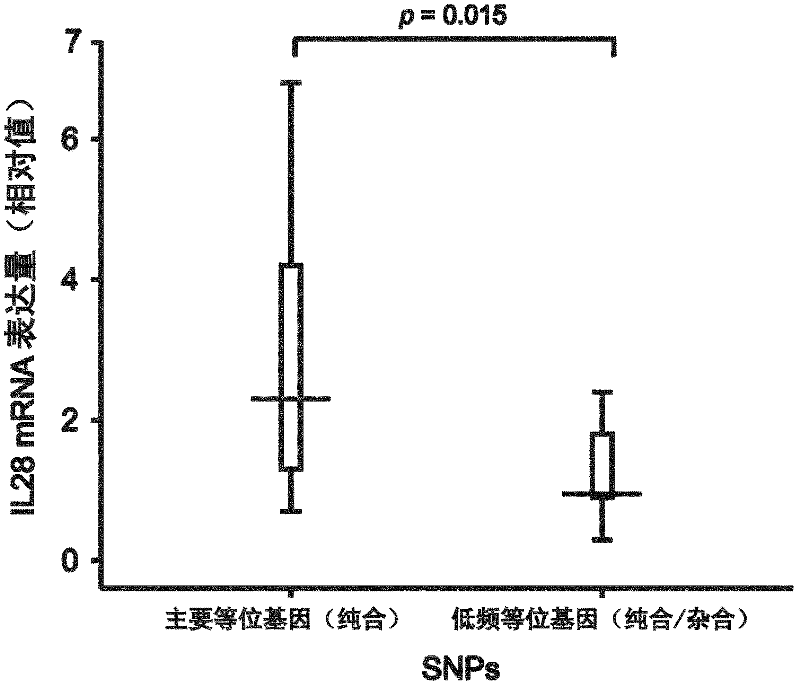
[0196] [Prediction of therapeutic effect on hepatitis C using GWAS]
[0197] Using Affymetrix Genome-Wide Human SNP Array 6.0 (hereinafter referred to as SNP Array 6.0) equipped with more than 900,000 probes for SNP analysis, 154 people who received PEG-IFN / RBV combination therapy for chronic hepatitis C were tested SNP typing, divided into subgroups according to drug sensitivity (GWAS stage (GWAS Stage): 1st panel (1st panel): 82 people in the ineffective (NVR) group and 72 people in the effective (VR) group), and a genome-wide association study ( GWAS).
[0198] will choose a P value of 10 -5 Table 1 shows the results in the case of the level or lower.
[0199] In addition, the P value was calculated mainly by chi-square test.
[0200] In addition, the ineffective group refers to a sample in which the virus did not disappear even once during the treatment.
[0201] It can be seen from the results that, especially for the low-frequency alleles of SNPs (rs12980275 and rs80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com