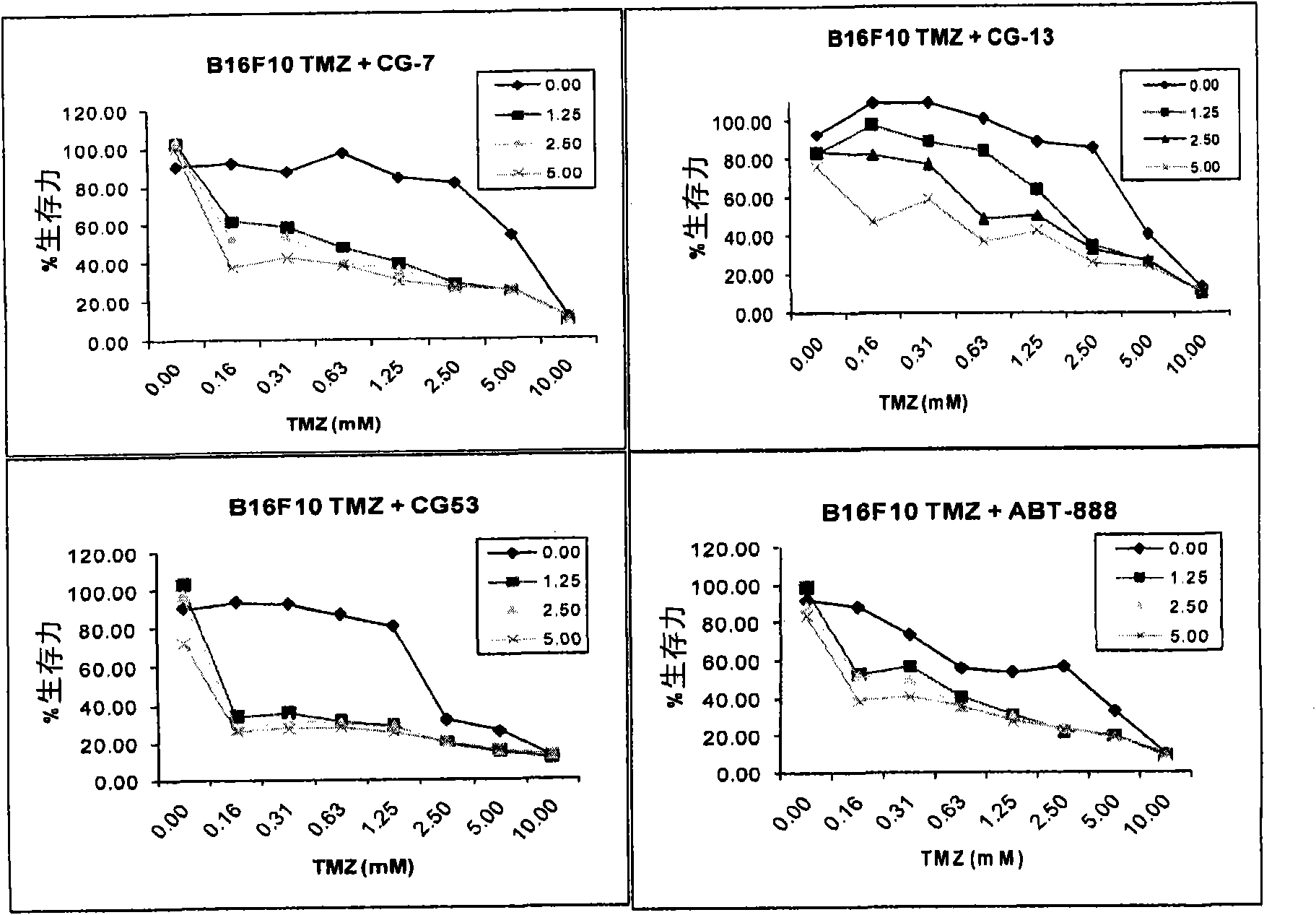
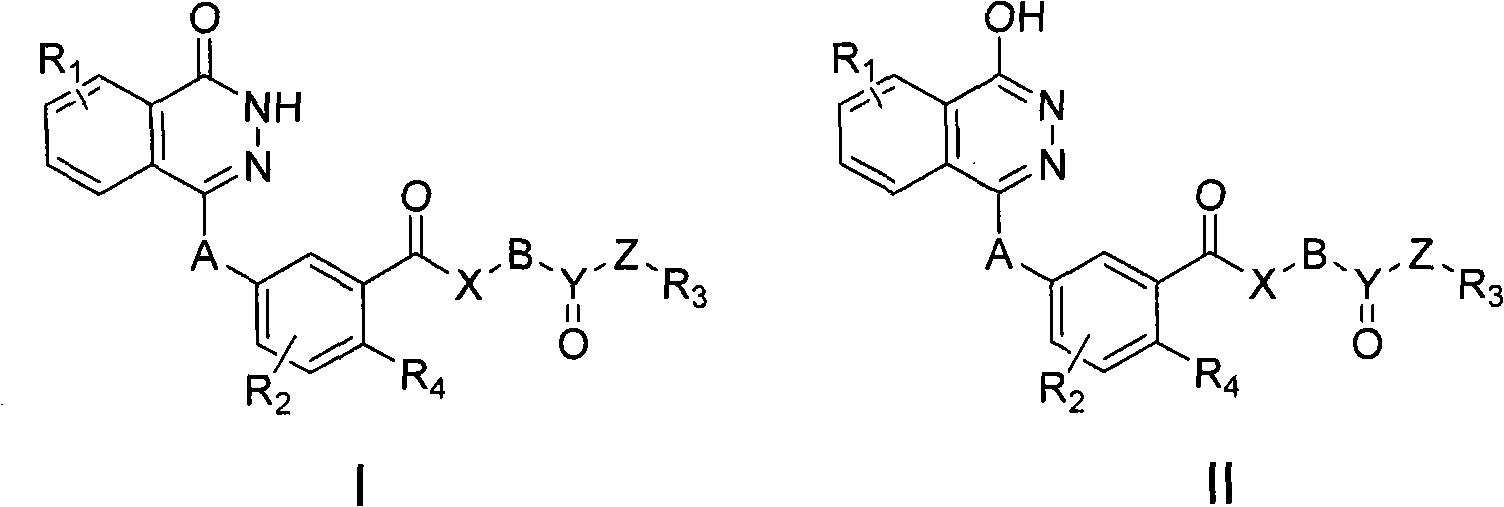
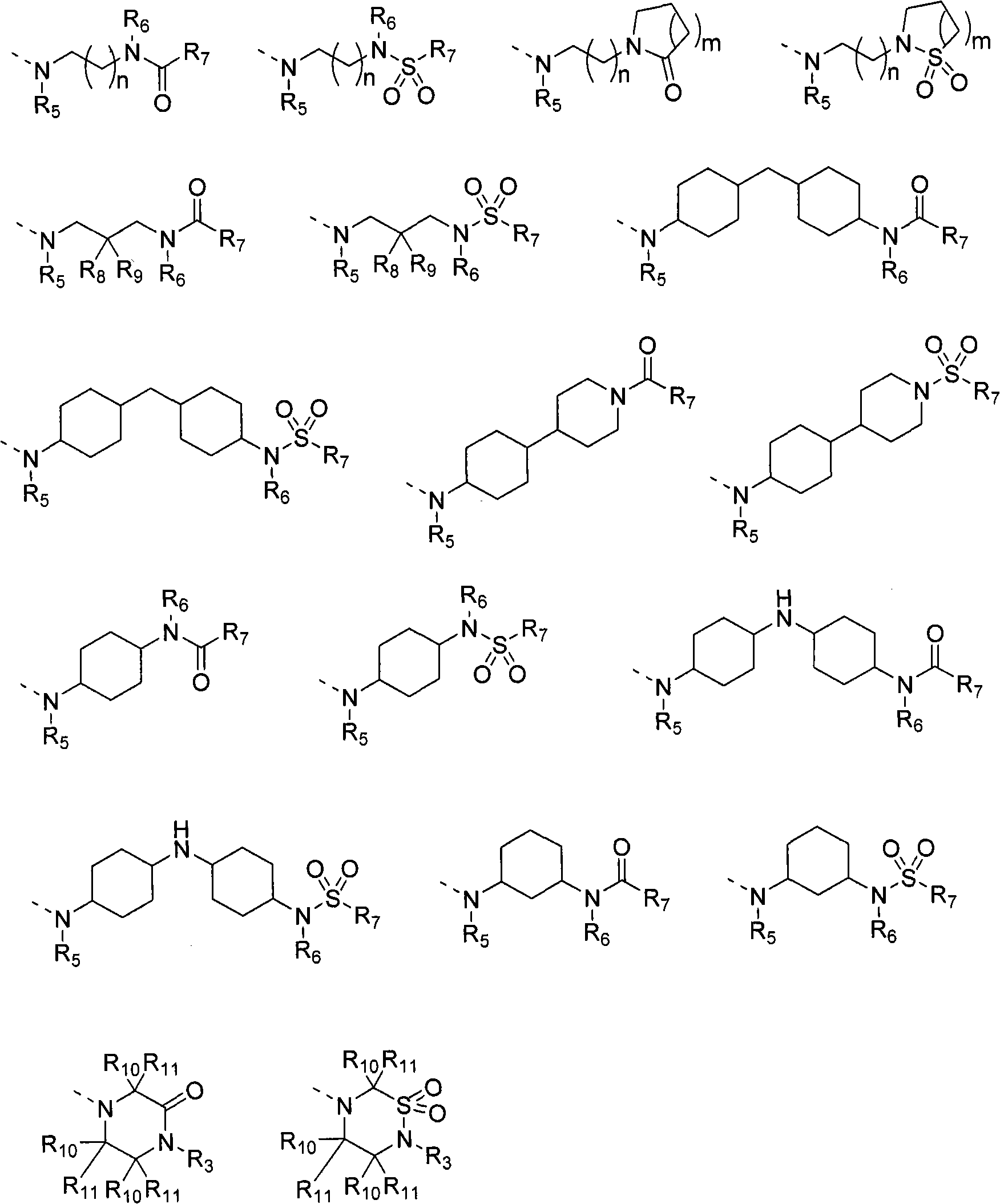
Substituted 2,3-phthalazinone compounds and application thereof
A compound and solvate technology, applied in the field of inhibiting the activity of poly(ADP-ribose) polymerase, can solve the problems of weakening the killing effect of cancer cells, weakening the killing effect of radiotherapy on cancer cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)-methyl)-N-(2-(2-oxotetrahydropyrrol-1-yl)ethyl base) benzamide
Embodiment 1A
[0172] Dimethyl (3-oxo-1,3-dihydroisobenzofuryl)phosphonate
[0173] Add 500 mL of anhydrous methanol to a 1 L pear-shaped flask, slowly add sodium block (11.5 g, 0.5 mol) under ice-bath conditions, and after it completely disappears, add diethyl phosphite (46 mL, 0.5 mol). After the mixture was stirred for 0.5 hour, o-carboxybenzaldehyde (52.5 g, 0.35 mol) was added, followed by warming to room temperature and stirring for 1 hour. Then methanesulfonic acid (36 mL, 0.55 mol) was added to the reactant, and the solvent was distilled off after stirring at room temperature for 2 hours, and the residue was dissolved in 400 mL of dichloromethane and 200 mL of water. The organic phase was washed sequentially with saturated sodium carbonate and saturated sodium chloride, then dried with anhydrous sodium sulfate, and filtered. After distilling off the solvent, the residue was recrystallized with a mixed solvent of dichloromethane and ether to obtain 64.25 g of white solid, yield: 75.8...
Embodiment 1B
[0175] 2-Fluoro-5-formyl-benzocyanide
[0176] Place 3-bromo-4-fluoro-benzaldehyde (100g, 0.49mol) in a 1L round bottom flask, dissolve it with 400 milliliters of NMP, then add cuprous cyanide (50.6g, 0.56mol), and heat the reactant to Stir overnight at 170°C. After cooling to room temperature, add an appropriate amount of diatomaceous earth, stir, and filter. The filtrate was separated with 400 ml of water and 500 ml of ethyl acetate, the organic phase was washed twice with water, dried with anhydrous sodium sulfate, filtered, and the solvent was distilled off. The residue was recrystallized from a mixed solvent of petroleum ether and ethyl acetate to obtain 55.95 g of a light yellow solid. Yield: 76.1%. MS (ESI): 150 (M+1, 100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com