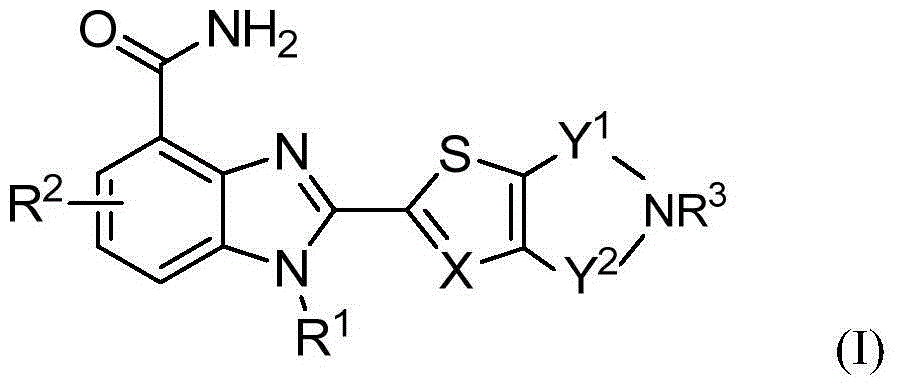
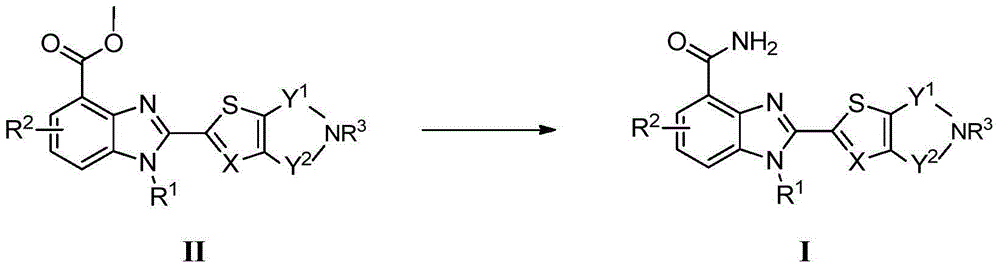
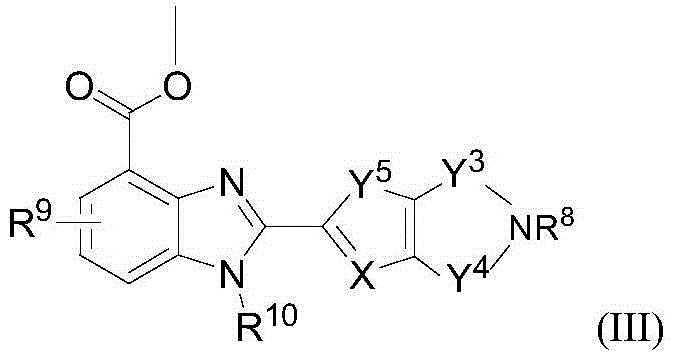
2-substituted-benzimidazole-4-formamide compound, preparation method, and application thereof
A compound, unsubstituted technology for medicinal chemistry and pharmacotherapeutics that addresses poor subtype selectivity and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1. 2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-carboxamide, I-1 Preparation of:
[0120] Step 1. Tert-butyl 6,7-dihydrothieno[3,2-c]pyridine-5(4H)-carboxylate:
[0121]
[0122] Weigh 4,5,6,7-tetrahydrothiophene[3,2-c]pyridine hydrochloride (13-1) (10g, 56.9mmol) in a reaction flask (500mL), add dichloromethane (300mL) . Triethylamine (8.7 mL) was added under ice-cooling, and a solution of di-tert-butyl dicarbonate (13.7 g, 62.8 mmol) in dichloromethane (20 mL) was added slowly. After the dropwise addition was completed, the reaction was tracked for 1 hour, and the reaction of the raw materials was complete. The reaction solution was washed with water and saturated NaCl aqueous solution successively, and the organic layer was retained, washed with anhydrous NaCl 2 SO 4 After drying, filtration and concentration under reduced pressure, the crude product was subjected to column chromatography (petroleum ether: ethyl acetate = 20:...
Embodiment 2
[0144] Example 2. 2-(5-Methyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-methanol Preparation of amides, 1-2:
[0145]Step 1. 2-(5-Methyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-carboxylic acid ester:
[0146]
[0147] 2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-carboxylic acid methyl ester (4-1)( 198mg, 0.63mmol) was dissolved in formic acid (3mL), and formaldehyde solution (37%, 0.3mL) was added. The reaction solution was raised to 95°C and reacted for 4 hours until the raw materials were completely reacted. Most of the solvent was evaporated under reduced pressure, and Na was added to the residue 2 CO 3 Adjust the pH to alkaline with a saturated aqueous solution, extract with ethyl acetate (50mL×3), wash the organic layer with water and saturated brine in turn, keep the organic layer, dry with anhydrous sodium sulfate, filter and concentrate under reduced pressure, and the crude product ...
Embodiment 3
[0153] Example 3. 2-(5-Ethyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-methanol Preparation of amides, 1-3:
[0154] Step 1. 2-(5-Ethyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-carboxylic acid ester:
[0155]
[0156] 2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)-1H-benzo[d]imidazole-4-carboxylic acid methyl ester (4-1)( 160mg, 0.51mmol) was dissolved in anhydrous DMF (5mL), added Cs 2 CO 3 (183mg, 0.56mmol) and ethyl bromide (67mg, 0.61mmol), the temperature was raised to 80°C for 1 hour. The reaction solution was poured into water, extracted with ethyl acetate (50mL×3), the organic layer was washed with water and saturated brine successively, the organic layer was retained, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and the crude product was subjected to column chromatography ( Dichloromethane:methanol=80:1) to obtain 150 mg of a light yellow gum with a yield of 86%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com