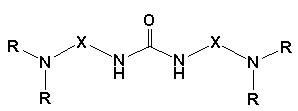
Compound containing tertiary amine derivatives, preparation method and application thereof
A technology containing tertiary amine urea and derivatives, applied in the field of fine chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
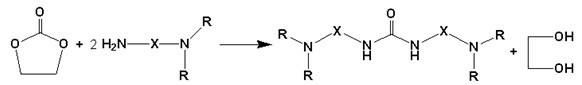
[0019] Add 2.2 g of ethylene carbonate into a 100 mL one-necked flask, and slowly add 5.8 g of N,N-diethylethylenediamine dropwise. Place the flask in an oil bath, connect a spherical condenser and a three-way joint, use a vacuum pump to evacuate the air in the system, and fill it with nitrogen as a protective gas. The temperature of the oil bath was raised to 120°C and the reaction was stirred at reflux. The reaction was stopped after 24 hours. After the product was cooled, the product was rotary evaporated at 90° C. for 1 hour under vacuum conditions to obtain an almost colorless transparent viscous liquid. The above liquid was analyzed by HPLC-MS combined technology, and the peak with a mass-to-charge ratio of 258, the molecular ion peak with a mass-to-charge ratio of 257, and the main fragment ion peak with a mass-to-charge ratio of 158 were detected in ESI-mode. The reactants and possible products of this reaction are analyzed and compared to determine that the product c...
Embodiment 2
[0021] Dissolve 4.2 grams of α, α'-dimethylol propionic acid in 86 grams of polyoxypropylene glycol and transfer to a 250 mL round bottom flask, then slowly add 29 grams of toluene diisocyanate dropwise, and stir the reaction at 80°C for 24 hours to make a prepolymer.
[0022] Weigh 4.2 g of [(C2H5)2NCH2CH2NH]2CO prepared in Example 1 and dissolve it in 220 g of pure water, add the above-mentioned prepolymer under stirring, and continue stirring for 4 hours to obtain a white emulsion. Add 22 grams of polyacrylic acid as a thickener to the emulsion under stirring, then add 60 grams of pure water, and stir for two hours to prepare the adhesive W. The adhesive adhesive layer has good flexibility and can be used for bonding between foam plastics.
Embodiment 3
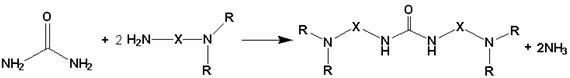
[0024] 1.5 g of urea was added into a 100 mL one-necked flask, and then 5.7 g of N-(2-aminoethyl)pyrrolidine was slowly added dropwise. Place the flask in an oil bath, connect a spherical condenser and a three-way joint, use a vacuum pump to evacuate the air in the system, and fill it with nitrogen as a protective gas. Raise the temperature of the oil bath to 140°C, stir and reflux the reaction, and use a vacuum pump to pump out the ammonia gas produced by the reaction after 2 hours, 4 hours, 8 hours, 18 hours, and 20 hours, and fill it in time. nitrogen. The reaction was stopped after 24 hours. After the product was cooled, the product was rotary evaporated at 90° C. for 1 hour under vacuum conditions to obtain a colorless transparent viscous liquid. The yield of the reaction under this condition was 88.3%. The above liquid was analyzed by HPLC-MS technology, and the peak with a mass-to-charge ratio of 254, the molecular ion peak with a mass-to-charge ratio of 253, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com