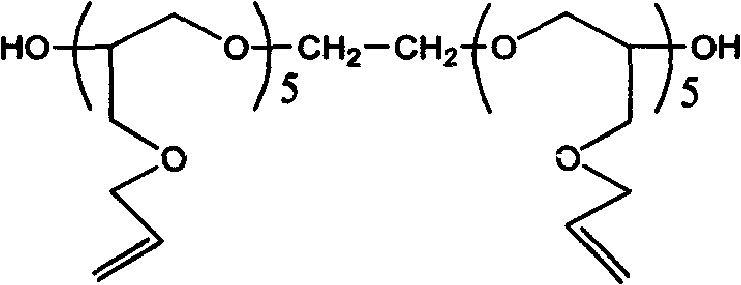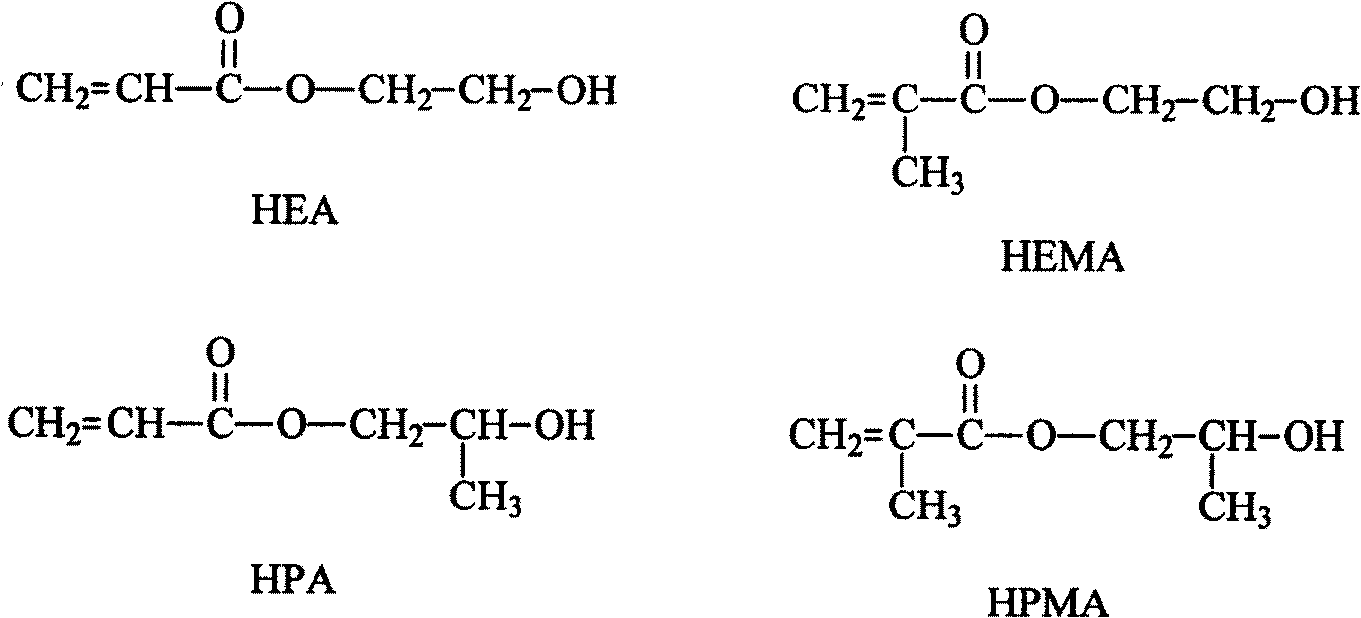Polyurethane acrylic acid ester and preparation method and application thereof
A polyurethane acrylate and acrylate technology, applied in polyurea/polyurethane coatings, coatings, etc., can solve problems such as heating, consumption, and unsuitability of heat-sensitive electronic circuit boards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
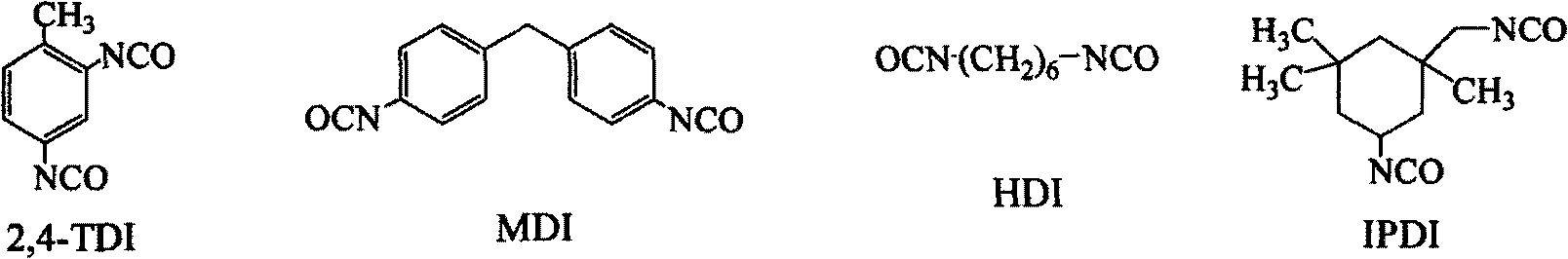
[0047] The raw material (II) hydroxyethyl methacrylate (HEMA) used in this embodiment, the raw material (III) is polyethylene glycol 400, the raw material (IV) is 2, 4 toluene diisocyanate, and the raw material dosage in this embodiment is the raw material (II): Raw material (III): Raw material (IV) = 1:1:2. The synthesis steps are as follows: add dehydrated raw material (III) into a dry three-necked round bottom flask, add dropwise raw material (IV) and dibutyltin laurate (catalyst) mixture under medium-speed stirring, (the dropping rate is 4 seconds / drop ). After the dripping is completed, the system is heated to 50°C, react for 4 hours until the isocyanate concentration does not change with time, and then slowly drip the raw material (II) into the system (the dripping rate is 4 seconds / drop). After the dripping is completed, The temperature of the system was raised to 80°C until the isocyanate concentration did not change with time, thereby obtaining the final product (I). ...
Embodiment 2
[0049] The amount of raw materials used in this example is raw material (II): raw material (III): raw material (IV): = 1:0:1, the synthesis step is: add raw material (IV) into a dry three-necked round bottom flask, and stir. Drop the mixture of raw material (II) and dibutyltin laurate (catalyst), (the dropping rate is 6 seconds / drop). After the dropping, the system is heated to 65°C and reacted for 5 hours until the isocyanate concentration does not change with time, so The final product (I) is obtained. The entire reaction is carried out in isolation of water (vapor) and under the protection of nitrogen or other non-reactive gases.
Embodiment 3
[0051] The raw material (IV) in this example is isophorone diisocyanate (IPDI), and the remaining raw materials and synthesis steps are the same as in Example 1. Compared with the TDI of Example 1, this example uses IPDI as the polyisocyanate component, the experimental conditions are better controlled, and the product storage stability is better, but the raw material price is slightly higher than that of TDI.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flexibility | aaaaa | aaaaa |
| Adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com