Method for preparing nano luminescent material ZnO/SnO2 heterostructure
A technology of nano-luminescent materials and heterostructures, which is applied in the fields of luminescent materials, nano-optics, nanotechnology, etc., can solve the problems that there are no reports on heterogeneous-structured nanowire luminescent materials, and achieve high product purity, easy operation, and controllable process strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
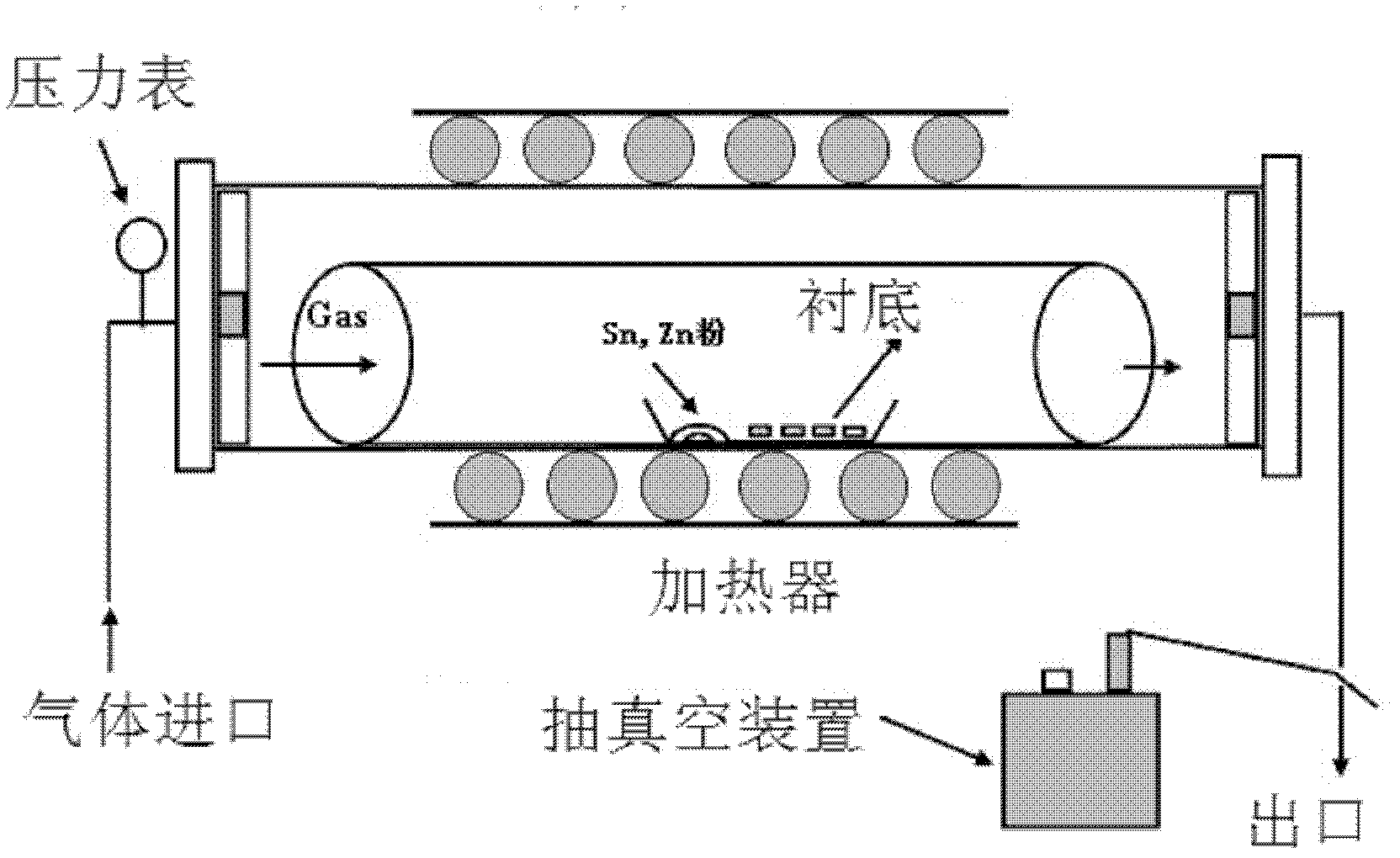
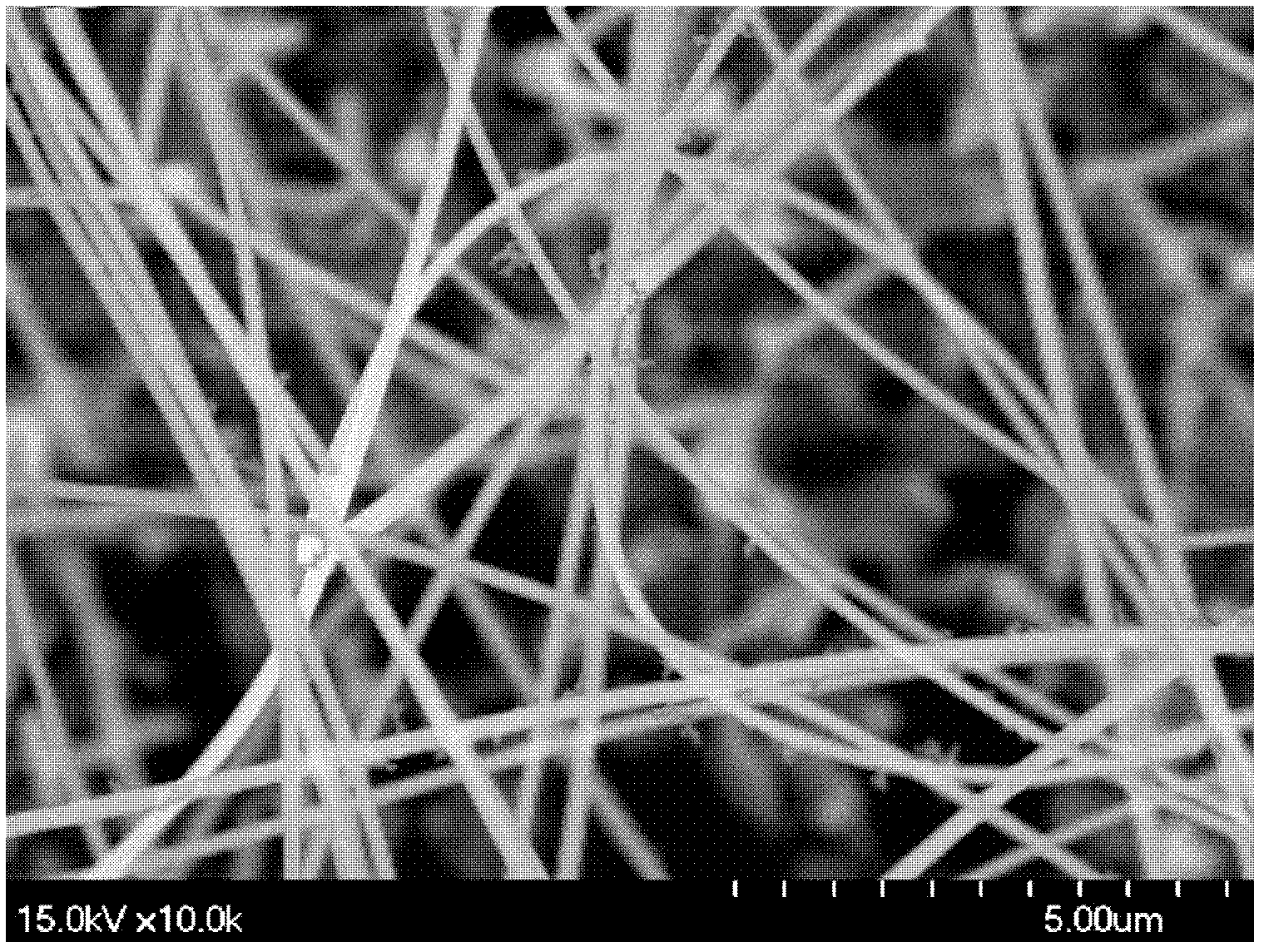
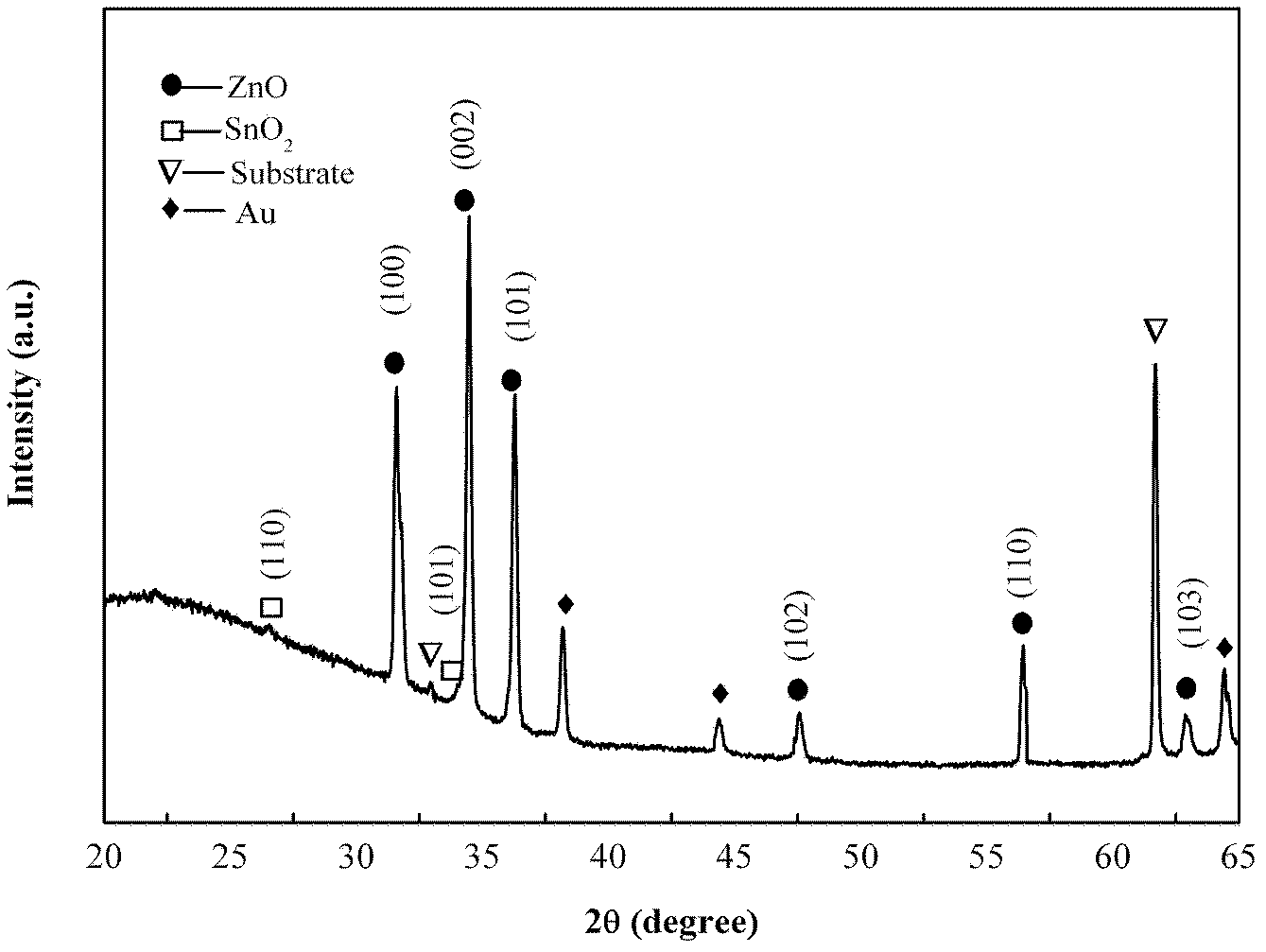
[0026] Weigh a certain amount of Sn powder and Zn powder with a molar ratio of 1:1 as the source material and place it at the front of the alumina boat, place a gold-plated silicon substrate 2 cm away from the source material, and then place the alumina boat in the middle of the tube furnace , add a refractory brick to each end of the pipe, cover (such as figure 1 ). Start the mechanical pump to make the air pressure in the tube reach -0.08MPa, close the valve, and introduce an appropriate amount of argon. When the pressure in the tube reaches an atmospheric pressure, open the valve to connect to the atmosphere, start the mechanical pump again and repeat the above operation four times to discharge the tube furnace cavity. air. Start the furnace, heat up to 950° C., feed 50 sccm (volume flow unit, meaning milliliters per minute under standard conditions) oxygen, and keep warm for 50 minutes. The furnace was naturally cooled to room temperature, the gas was turned off, the sub...
Embodiment 2
[0028] As in Example 1, take a certain amount of Sn powder and Zn powder with an electronic balance as the source material and place it on the front of the alumina boat, place a gold-plated silicon substrate at a distance of 2 cm from the source material, and then place the alumina boat on the tube furnace In the middle, a refractory brick is added to each end of the pipe, and the cover (such as figure 1 ). Start the mechanical pump to make the air pressure in the tube reach -0.08MPa, close the valve, and introduce an appropriate amount of argon. When the pressure in the tube reaches an atmospheric pressure, open the valve to connect to the atmosphere, start the mechanical pump again and repeat the above operation four times to discharge the tube furnace cavity. air. Start the furnace, heat up to 950°C, feed 50 sccm of oxygen, and keep the temperature for 70 minutes. The furnace was naturally cooled to room temperature, the gas was turned off, the substrate was taken out, an...
Embodiment 3
[0030] As in Example 1, take a certain amount of Sn powder and Zn powder with an electronic balance as the source material and place it on the front of the alumina boat, place a gold-plated silicon substrate at a distance of 2 cm from the source material, and then place the alumina boat on the tube furnace In the middle, a refractory brick is added to each end of the pipe, and the cover (such as figure 1 ). Start the mechanical pump to make the air pressure in the tube reach -0.08MP, close the valve, and introduce an appropriate amount of argon gas. When the pressure in the tube reaches an atmospheric pressure, open the valve to connect to the atmosphere, start the mechanical pump again and repeat the above operation four times to discharge the tube furnace cavity. air. Start the furnace, heat up to 1050°C, feed 50 sccm of oxygen, and keep the temperature for 50 minutes. The furnace was naturally cooled to room temperature, the gas was turned off, the substrate was taken out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com