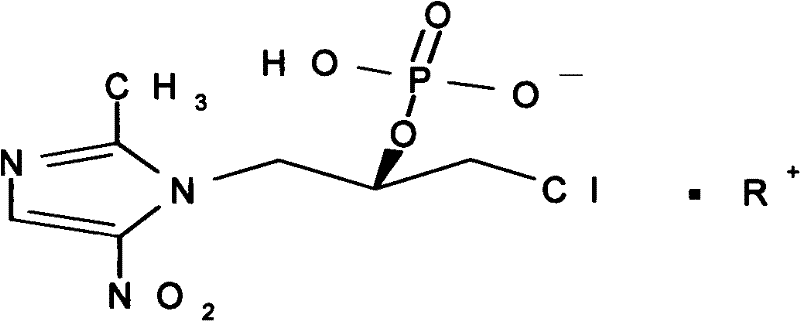
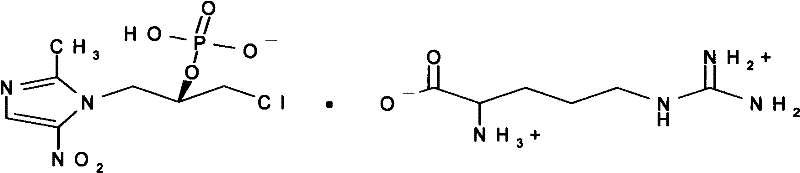
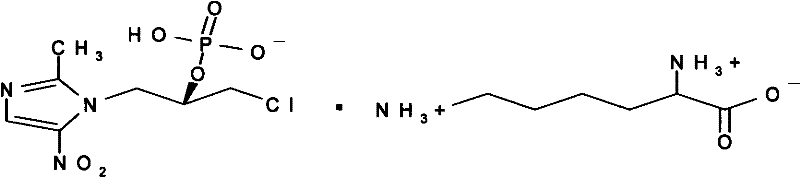
S-Ornidazole phosphate amino acid salt, its preparation method and application
A technology of levo-ornidazole phosphate and amino acid, which is applied in the field of levo-ornidazole phosphate amino acid salt and its preparation, can solve the problems of unsafe, poor water solubility and low melting point of levo-ornidazole, and achieve good solubility and superior The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of L-Ornidazole Phosphate Monoarginine Salt
[0030] Dissolve 100g of L-ornidazole phosphate in 500ml of methanol at 50°C, slowly add 58.2g of arginine under stirring, stir for 2 hours until the solids are completely dissolved, continue stirring for 1 hour, filter, evaporate to dryness under reduced pressure, add Dissolve the residue in 1800ml of 90% acetone solution at 60°C, decolorize with 0.5% activated carbon for 15min, filter, and place the filtrate for crystallization to obtain 113g of L-ornidazole phosphate monoarginine salt. (Purity 99.2%, calculated by normalization method).
Embodiment 2
[0031] Embodiment 2: Preparation of L-Ornidazole Phosphate Monolysine Salt
[0032] The preparation method is the same as in Example 1, except that arginine is replaced by lysine.
[0033] Embodiment 2: Preparation of L-Ornidazole Phosphate Monohistidine Salt
[0034] The preparation method is the same as in Example 1, except that arginine is replaced by histidine.
Embodiment 4
[0035] Example 4: Preparation of L-Ornidazole Phosphate Monoarginine Salt for Injection
[0036] Levonidazole Phosphate Monosperm 250g
[0037] Amino acid salt
[0038] Water for injection 2000ml
[0039] Makes 1000 bottles
[0040] Add 1900ml of water for injection in the liquid preparation container, add the prescribed amount of L-ornidazole phosphate monoarginine salt, stir to dissolve, add water to the full amount, then add 0.05% (W / V) medicinal charcoal, stir After 60 minutes, filter with a 0.22 μm filter membrane until the clarity is acceptable. After the content of intermediates is determined to be qualified, the fixed amount is divided into control vials, half stoppered, and the sample is pre-frozen at -50°C for 4 hours, and vacuumized. Keep the vacuum degree <20Pa, slowly raise the temperature to -20°C, keep it for 20 hours, then slowly raise the temperature to 20°C, keep it for 4 hours, the pressure recovery test is less than 30Pa / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com