Preparation method of mutant of double-carbonyl reductase containing D-amino acid
A D-type, aminoacyl technology, applied in the field of preparing proteins and/or polypeptides containing D-type amino acids, can solve problems such as unsatisfactory, and achieve the effect of low cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
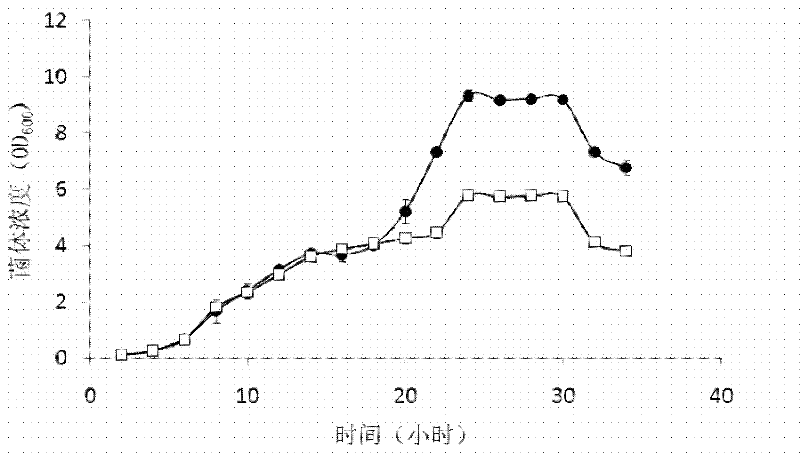
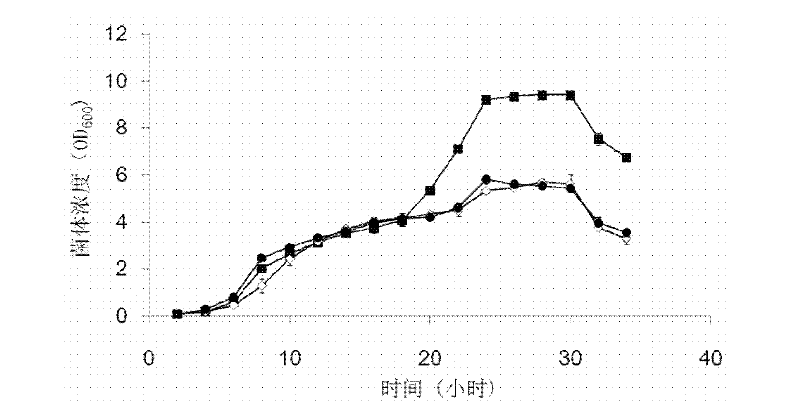
[0040] Example 1: Effect of D-lysine on the growth of transformed bacteria containing pAC-ph△-AK3
[0041] Firstly, an E. coli prokaryotic expression system was prepared, which contained pAC-ph△-AK3 plasmid with p15A replicon and pETDuet-T2 with ColE1 replicon D K-DKR plasmid.
[0042] In the above technical scheme, the pAC-ph△-AK3 plasmid is transformed from pACYC184, with a p15A replicon: the sequence from position 2920 to position 3542 of pACYC184 is replaced by pH In the sequence of aminoacyl tRNA synthetase, the sequence from position 1425 to position 1524 is replaced by three inhibitory tRNA sequences. pAC-ph△-AK3 can code from Pyrococcus horikoshii Lysyl tRNA synthetase and inhibitory tRNA (tRNA Lys ) molecular pair, in which lysyl tRNA synthetase is regulated by glutamine tRNA synthetase promoter and terminator, and suppressor tRNA is regulated by lpp promoter and rrnC terminator; in addition, chloramphenicol acetyl transfer The 112th aspartic acid codon of the e...
Embodiment 2
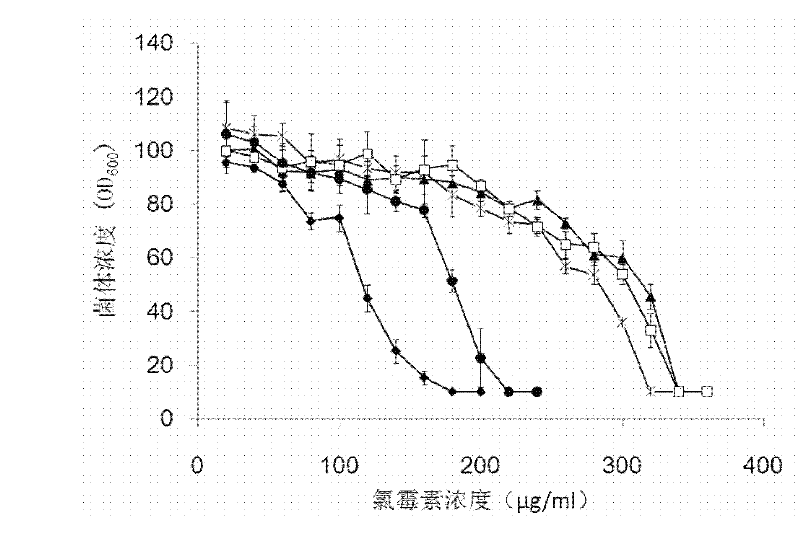
[0048] Example 2: Effect of D-lysine on the chloramphenicol tolerance of pAC-phΔ-AK3-containing transformed bacteria.
[0049] Transfect the pAC-ph△-AK plasmid into Escherichia coli BL21 (DE3) competent cells, spread it on a plate containing 4 μg / ml tetracycline and 20 μg / ml tetracycline, incubate at 37°C for about 30 hours, pick the co-transformation plate A single colony was cultured in LB liquid medium with tetracycline and chloramphenicol for 20 hours, and the LB culture solution was diluted into M9 medium with tetracycline and chloramphenicol to make its OD 600 About 0.2, cultured at 37°C for about 30 hours, under D-lysine with different concentration gradients (1.2, 2.4, 3.6, 5mM), transferred to 0, 20, 40, 60, 80, 100, 120, 140 , 160, 180, 200, 220, 240, 260, 280, 300, 320, 340, 360 μg / ml of chloramphenicol concentration gradient in the M9 medium, which does not contain chloramphenicol as the control group. Measure the OD after culturing for about 30 hours 600 value, ...
Embodiment 3
[0050] Example 3: Expression and purification of D-type lysine double carbonyl reductase mutant
[0051]The site-directed introduction system of D-lysine is composed of pAC-ph△-AK3 and pETDuet-T2 D K-DKR two plasmids co-transformed Escherichia coli BL21 (DE3) constituted. The two plasmids contain compatible replicons p15A and ColE1 respectively, and the pAC-ph△-AK3 code is derived from Pyrococcus horikoshii Lysyl tRNA synthetase ( pH tRNARS) and inhibitory tRNA, pETDuet-T2 D K-DKR contains a mutant gene of double carbonyl reductase, in which pETDuet-T2 D The K-DKR plasmid contains the following four features: (1) The second codon of the gene encoding the double carbonyl reductase is mutated from ACC to the amber codon TAG, (2) His6 is added to the N-terminal of the double carbonyl reductase mutant gene (3) The mutant gene of double carbonyl reductase was constructed into the first multiple cloning site of pETDuet-1, and its expression was regulated by the lactose operon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com