In vitro amplification and low-temperature storage method for regulatory T cells of umbilical cord blood
A technology for cryopreservation and umbilical cord blood, applied in the field of cell expansion and preservation in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 6% hydroxyethyl starch substitute slurry to cord blood at a volume ratio of 4:1, place in a refrigerator at 4°C for 1 hour to precipitate red blood cells, take the upper plasma layer and centrifuge (2000rpm / min, 5min), discard most of the plasma, and precipitate white blood cells. After suspension, they were separated with Ficoll lymphocyte separation medium (2000rpm / min, 20min), and washed three times with PBS to obtain umbilical cord blood mononuclear cells. According to the manufacturer's instructions, use standard separation kits, such as CliniMACS, Miltenyi Biotec's anti-human CD8, CD14, CD19, CD56 monoclonal antibody immunomagnetic beads to remove non-CD4 cells in the cord blood mononuclear cells, and then isolate the cord blood CD4+ T cells Enrich CD4+CD25+ cells with anti-human CD25 antibody-coupled magnetic beads. After the cord blood CD4+CD25+T cells were purified, the purity of the isolated cells was detected by flow cytometry.
Embodiment 2
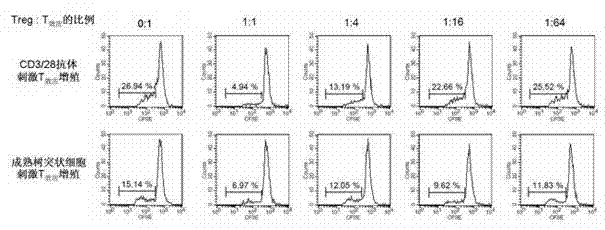
[0036] In vitro expansion of purified cord blood CD4+CD25+ T cells with anti-human CD3 / CD28 antibody-coupled magnetic beads and recombinant human IL-2 (200 U / ml) in commercially available cell culture bags or cell culture plates , using X-VIVO15 complete culture medium (containing 10% inactivated human AB serum, penicillin and streptomycin double antibody 50U / ml), the ratio of CD3 / CD28-coated Dynal Beads to cells in the culture system is 1:1 , at 37℃, 5%CO 2 Cultured under the conditions of humidity and saturated humidity, half of the culture medium and IL-2 were replaced every other day. The whole expansion time is 2-3 weeks, including two rounds of expansion of 6-8 days each and two rounds of expansion in the middle of which the cells rest for 2-4 days, that is, after the cells are removed from the magnetic beads, the cells contain IL-2 (20U / ml ) of X-VIVO15 complete culture medium for 2 days. After the in vitro expansion, the number of cells increased by about 1500 times,...
Embodiment 3
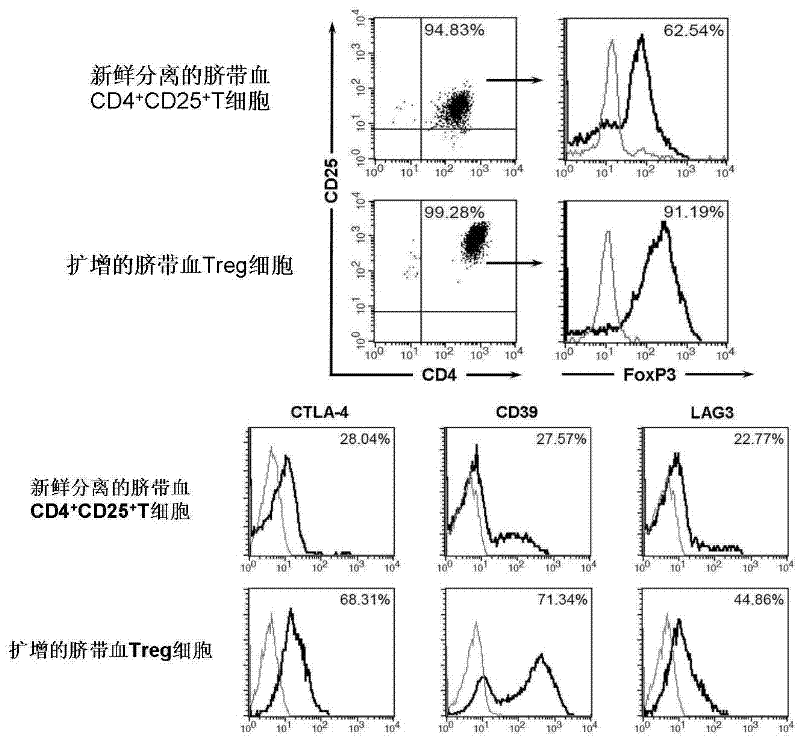
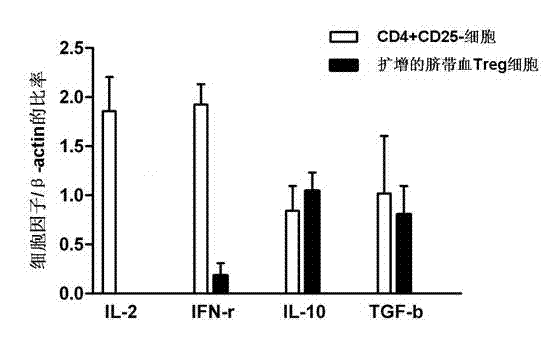
[0038] Immunophenotyping of cord blood Treg cells was performed by flow cytometry. Use FITC-labeled CD4 antibody, PE-Cy5-labeled CD25 antibody, APC-labeled CD127 antibody, PE-labeled CTLA-4 antibody, CD39 antibody, and LAG3 antibody for cell surface labeling. PE-tagged Foxp3 for intracellular labeling. Such as figure 1 As shown, the expanded cord blood Treg cells co-expressed CD4 and CD25, and more than 90% of the cells expressed FoxP3. At the same time, with freshly sorted CD4 + CD25 + Compared with T cells, expanded cord blood Treg cells highly expressed CTLA-4 (68%), CD39 (71%) and LAG-3 (45%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com