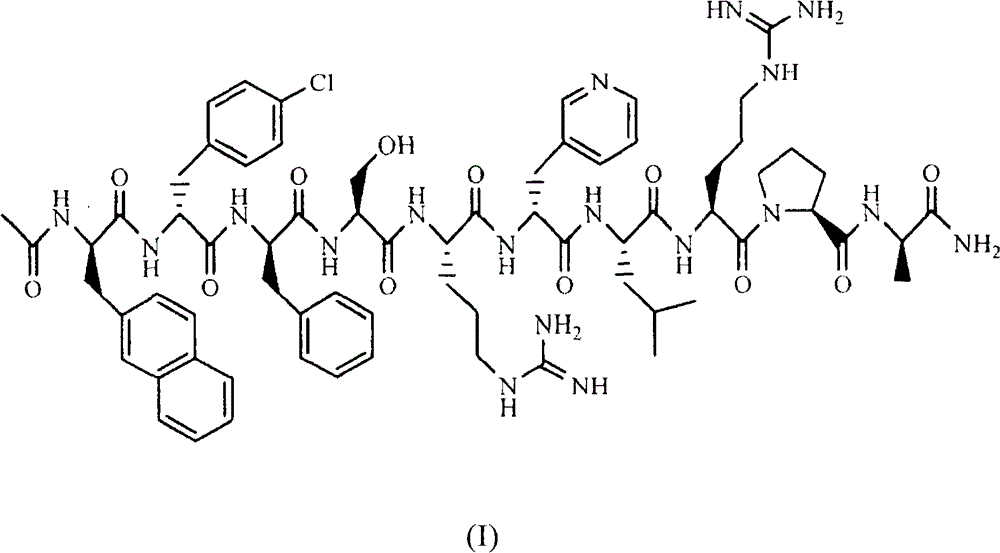
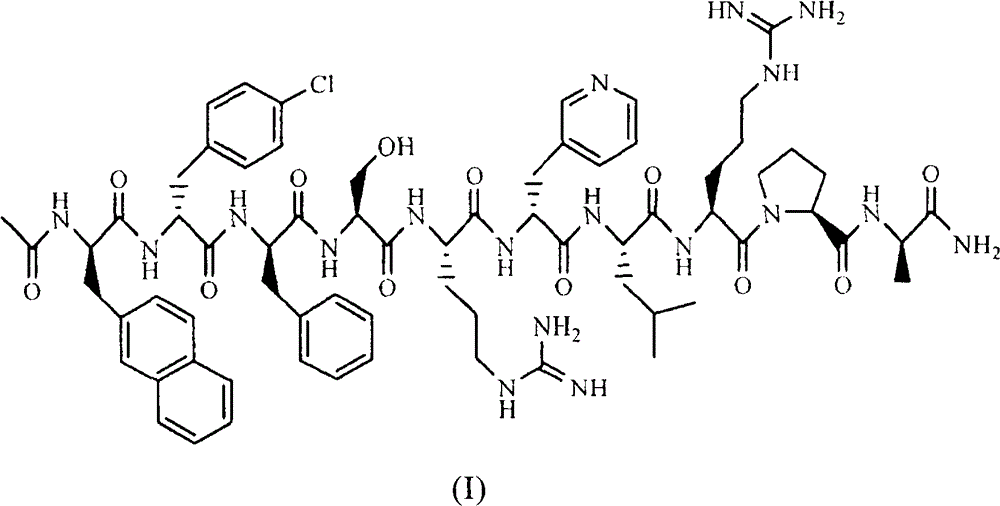
Sustained-release preparation for injection of lhrh antagonist substances and preparation thereof
A technology for sustained-release preparations and injections, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc., and can solve the problems of long course of treatment, short half-life, patient pain and inconvenience of medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] Preparation method of sustained-release preparation for injection
[0067] In another aspect, the preparation method of the sustained-release preparation for injection of the present invention is provided, the method comprising the following steps:
[0068] (a) dispersing a compound of formula (I) or a pharmaceutically acceptable salt thereof in an aqueous solvent to obtain an aqueous dispersion;
[0069] (b) dispersing acidic phospholipids or pharmaceutically acceptable salts thereof and neutral phospholipids in an aqueous solvent to obtain an aqueous dispersion;
[0070] (c) uniformly mixing the aqueous dispersion obtained in step (a) with the aqueous dispersion obtained in step (b);
[0071] (d) drying the mixed solution obtained in step (c) into a solid; and
[0072] (e) dispersing the solid obtained in step (d) in an oily solvent.
[0073] As used herein, the term "dispersion" refers to the distribution of one or more substances (ie, dispersoid) in another sub...
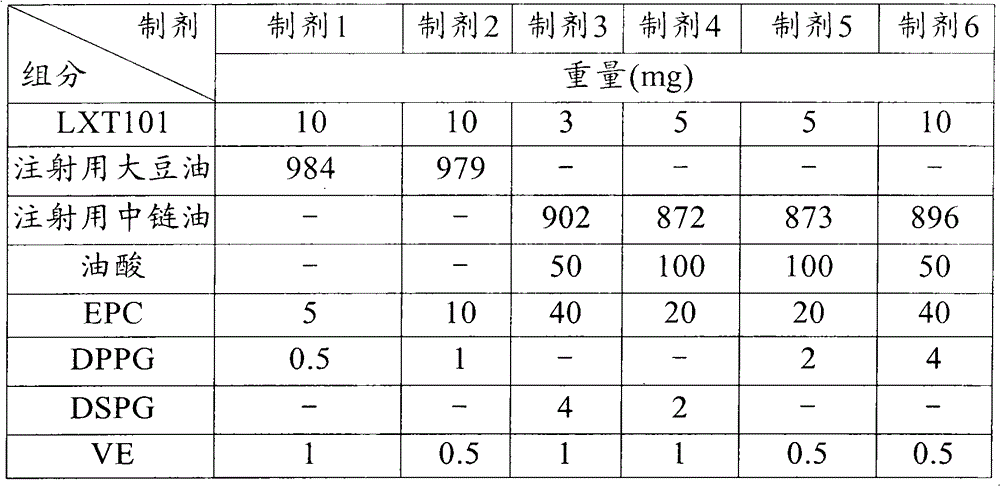
Embodiment 1
[0092] Embodiment 1: Preparation of sustained-release preparation for injection
[0093] Materials and Reagents
[0094] active pharmaceutical ingredient
[0095] LXT101: synthesized by the inventor's laboratory according to the method of Chinese patent ZL90108955.9.
[0096] oily solvent
[0097] Medium-chain oil for injection and soybean oil for injection: both were purchased from China Tieling Beiya Pharmaceutical Oil Co., Ltd.
[0098] other reagents
[0099] Vitamin E (VE): purchased from Zhejiang NHU Co., Ltd.;
[0100] Dipalmitoylphosphatidylcholine (DPPG), distearoylphosphatidylcholine (DSPG) and egg yolk lecithin (EPC): were purchased from China Shanghai Dongshang Industrial Co., Ltd.;
[0101] Oleic acid: purchased from Shanghai Dongshang Industrial Co., Ltd.
[0102] pure distilled water
[0103] method
[0104] The preparation method comprises the following steps:
[0105] (a) Disperse LXT101 in pure distilled water;
[0106] (b) DPPG or DSPG an...
Embodiment 2
[0114] Example 2: Results of pharmacodynamic studies in rats
[0115] Six sustained-release preparations for LXT101 injection prepared in Example 1 were injected intramuscularly (i.m) into rats. Testosterone content (ng / ml) was measured at different times (days) after administration to indirectly measure the sustained-release effect of the LXT101 preparation in vivo. The whole determination process is completed by using BECKMANCOULTERAccessImmunoassaySystem chemiluminescence instrument. The results fit in Table 2 below.
[0116] As mentioned above, the sustained-release effect of the LXT-101 preparation was evaluated by measuring the testosterone level in rats. As shown in Table 2, when the dose is 3.5 mg / kg, preparation 1 can continuously suppress testosterone to castrate level for 5 to 7 days; when the dose is 3.5 mg / kg, preparation 2 can continuously suppress testosterone to castrate level The time of castration level is 7 to 9 days; when the dose is 3.5mg / kg, the time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com