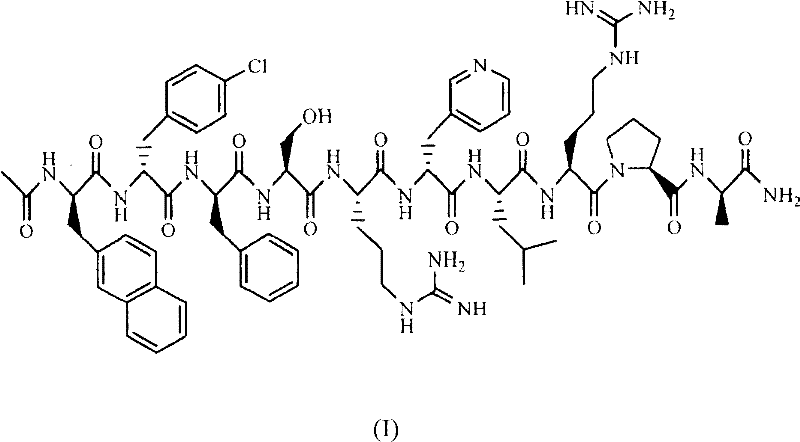
Injection sustained-release preparation of LHRH (luteinizing hormone releasing hormone) antagonist substance and preparation thereof
A technology for sustained-release preparations and injections, which can be applied to non-active ingredients medical preparations, medical preparations containing active ingredients, and drug combinations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] Preparation method of sustained-release preparation for injection
[0067] In another aspect, the preparation method of the sustained-release preparation for injection of the present invention is provided, the method comprising the following steps:
[0068] (a) dispersing a compound of formula (I) or a pharmaceutically acceptable salt thereof in an aqueous solvent to obtain an aqueous dispersion;
[0069] (b) dispersing acidic phospholipids or pharmaceutically acceptable salts thereof and neutral phospholipids in an aqueous solvent to obtain an aqueous dispersion;
[0070] (c) uniformly mixing the aqueous dispersion obtained in step (a) with the aqueous dispersion obtained in step (b);
[0071] (d) drying the mixed solution obtained in step (c) into a solid; and
[0072] (e) dispersing the solid obtained in step (d) in an oily solvent.
[0073] As used herein, the term "dispersion" refers to the distribution of one or more substances (ie, dispersoid) in another sub...
Embodiment 1
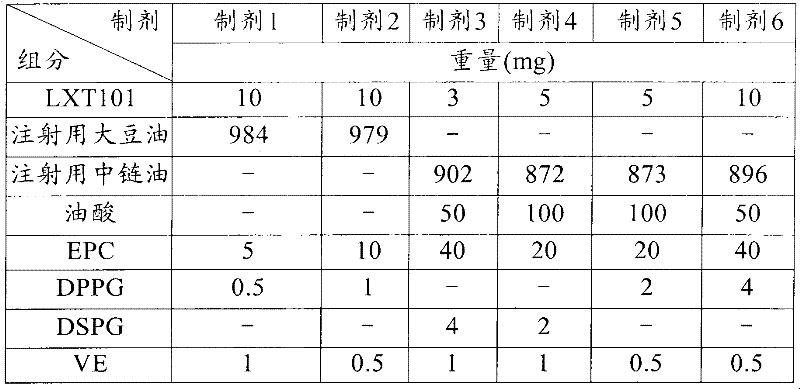
[0092] Embodiment 1: Preparation of sustained-release preparation for injection
[0093] Materials and Reagents
[0094] active pharmaceutical ingredient
[0095] LXT101: Synthesized by the inventor's laboratory according to the method of Chinese patent ZL 90108955.9.
[0096] oily solvent
[0097] Medium-chain oil for injection and soybean oil for injection: both were purchased from China Tieling Beiya Pharmaceutical Oil Co., Ltd.
[0098] other reagents
[0099] Vitamin E (VE): purchased from Zhejiang NHU Co., Ltd.;
[0100] Dipalmitoylphosphatidylcholine (DPPG), distearoylphosphatidylcholine (DSPG) and egg yolk lecithin (EPC): were purchased from China Shanghai Dongshang Industrial Co., Ltd.;
[0101] Oleic acid: purchased from Shanghai Dongshang Industrial Co., Ltd.
[0102] pure distilled water
[0103] method
[0104] The preparation method comprises the following steps:
[0105] (a) Disperse LXT101 in pure distilled water;
[0106] (b) DPPG or DSPG a...
Embodiment 2
[0114] Example 2: Results of pharmacodynamic studies in rats
[0115] Six sustained-release preparations for LXT101 injection prepared in Example 1 were injected intramuscularly (i.m) into rats. Testosterone content (ng / ml) was measured at different times (days) after administration to indirectly measure the sustained-release effect of the LXT101 preparation in vivo. The entire determination process was completed using a BECKMAN COULTER Access Immunoassay System chemiluminescence instrument. The results fit in Table 2 below.
[0116] As mentioned above, the sustained-release effect of the LXT-101 preparation was evaluated by measuring the testosterone level in rats. As shown in Table 2, when the dose is 3.5 mg / kg, preparation 1 can continuously suppress testosterone to castrate level for 5 to 7 days; when the dose is 3.5 mg / kg, preparation 2 can continuously suppress testosterone to castrate level The time of castration level is 7 to 9 days; when the dose is 3.5mg / kg, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com