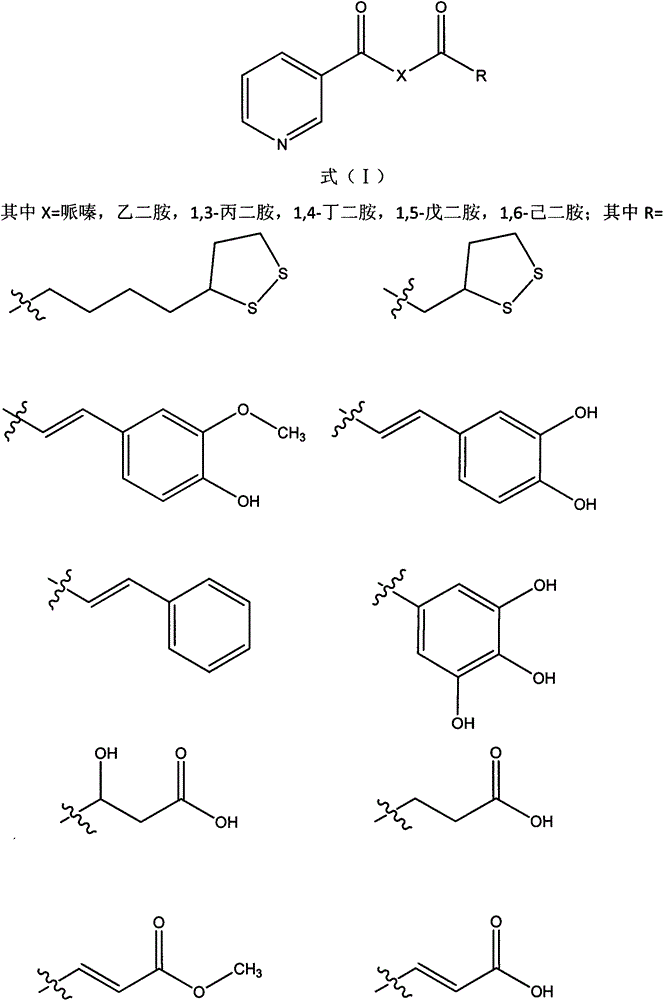
Nicotinic acid derivative, its preparation method and its pharmaceutical composition
A technology of drugs and compounds, applied in the field of medicine, can solve the problems of large therapeutic dose, limited clinical application, induced ulcer disease and gout, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
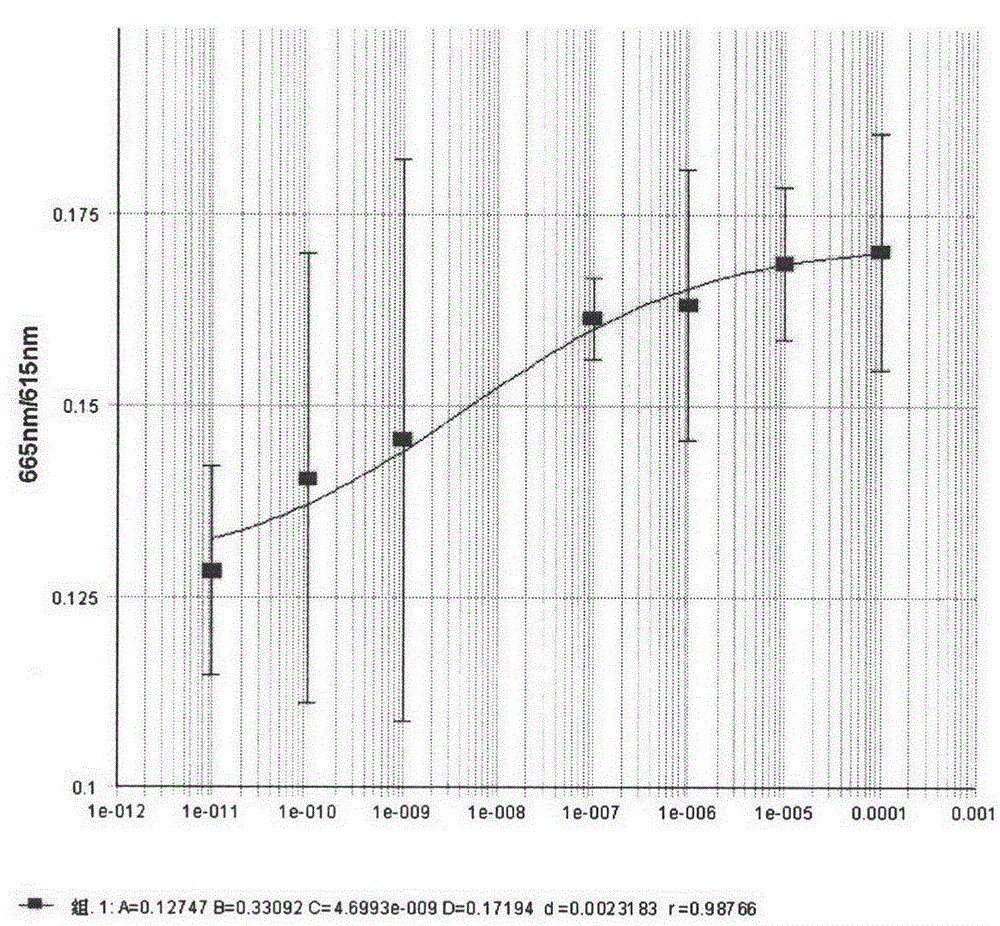
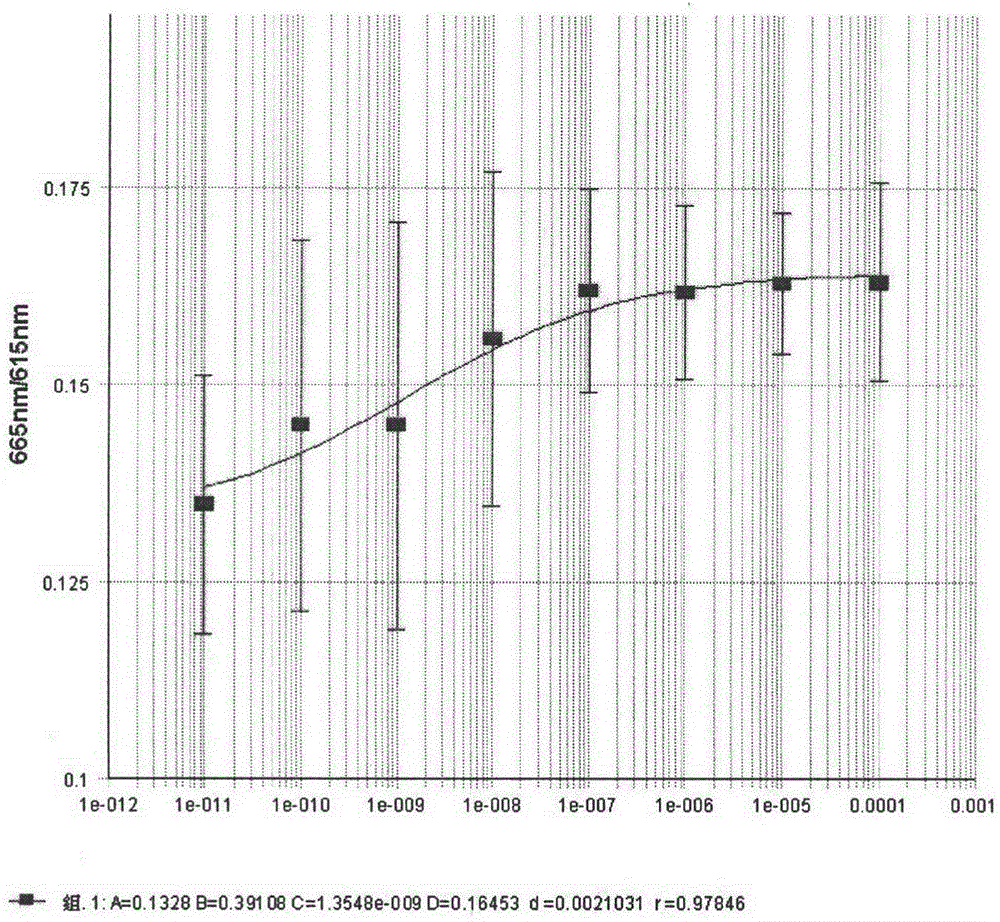
Image
Examples
Embodiment 1
[0041]
[0042] N-(2-(5-(1,2-dithiolan-3-yl)pentanamido)ethyl)nicotinamide
[0043] Dissolve lipoic acid (6.18g, 30mmol) and BOP (15.9g, 36mmol) in 48ml of dichloromethane, add ethylenediamine (120mmol, 8.01ml) dropwise in an ice bath environment, stir for 60 minutes, then at room temperature Reacted for 30 minutes, the system was extracted three times with 20ml of saturated brine each time, and once with water, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product, which was dissolved in 60ml of dichloromethane, and 4.2ml of TEA and niacin (1.11g, 9mmol) were added. With BOP (4.38g, 9.9mmol), the reaction was stirred at room temperature, monitored by TLC until the reaction was complete, and then extracted three times with 60ml of saturated brine each time, once with water, dried over anhydrous magnesium sulfate, concentrated to obtain the crude product, silica gel column Chromatographic separation, eluent methanol: dichloromethane = 1...
Embodiment 2
[0045]
[0046] N-(3-(5-(1,2-dithiolan-3-yl)pentanamido)propyl)nicotinamide
[0047] Dissolve lipoic acid (1.03g, 5mmol) and BOP (2.65g, 6mmol) in 16ml of dichloromethane, drop them into 1,3-propanediamine (3.40ml, 40mmol) in an ice bath, and stir for 60 Minutes, then react at room temperature for 30 minutes. The system is extracted three times with 20ml saturated brine each time, and once with water, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product, which is dissolved in 20ml dichloromethane, and 1.4ml TEA, niacin (0.37 g, 3mmol) and BOP (1.46g, 3.3mmol). The reaction was stirred at room temperature. The reaction was monitored by TLC until the reaction was complete. Then, it was extracted three times with 20ml of saturated brine and once with water, dried over anhydrous magnesium sulfate, and concentrated to obtain a crude The product was separated by silica gel column chromatography, eluent methanol: dichloromethane = (1:100-5:1...
Embodiment 3
[0049]
[0050] N-(6-(5-(1,2-dithiolan-3-yl)pentanamido)hexyl)nicotinamide
[0051] Lipoic acid (1.03g, 5mmol) and BOP (2.65g, 6mmol) were dissolved in 16ml of dichloromethane, and added dropwise to 1,6-hexanediamine (5.27ml, 40mmol) in an ice bath, and stirred for 60 Minutes, then react at room temperature for 30 minutes. The system is extracted three times with 20ml saturated brine each time, and once with water, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product, which is dissolved in 20ml dichloromethane, and 1.4ml TEA, niacin (0.37 g, 3mmol) and BOP (1.46g, 3.3mmol). The reaction was stirred at room temperature. The reaction was monitored by TLC until the reaction was complete. Then, it was extracted three times with 20ml of saturated brine and once with water, dried over anhydrous magnesium sulfate, and concentrated to obtain a crude The product was separated by silica gel column chromatography, eluent methanol: dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com