Ultraviolet quantitative determination method for concentration of electrolyte of positive electrode of vanadium battery and application thereof
A cathode electrolyte, quantitative determination technology, applied in the measurement of color/spectral characteristics, etc., can solve the problems of cumbersome operation process and insufficient accuracy of vanadium ion concentration, and achieve the effect of simple operation process, accurate and reliable analysis results, and avoiding interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The ultraviolet quantitative determination method of the positive electrode electrolyte concentration of the vanadium battery of the present embodiment comprises the following steps:
[0032] 1. Determine the number of vanadyl sulfate crystal water by thermogravimetric method, and use it as V (IV) standard sample.
[0033] 2. In the wavelength range of 190nm to 900nm, scan the spectrum of the sulfuric acid solution, the concentration of the sulfuric acid solution is 1mol / L. It is determined that the sulfuric acid solution has no characteristic absorption peak in the wavelength range of 190nm to 900nm, which will not interfere with the determination of the absorbance of vanadium ions.
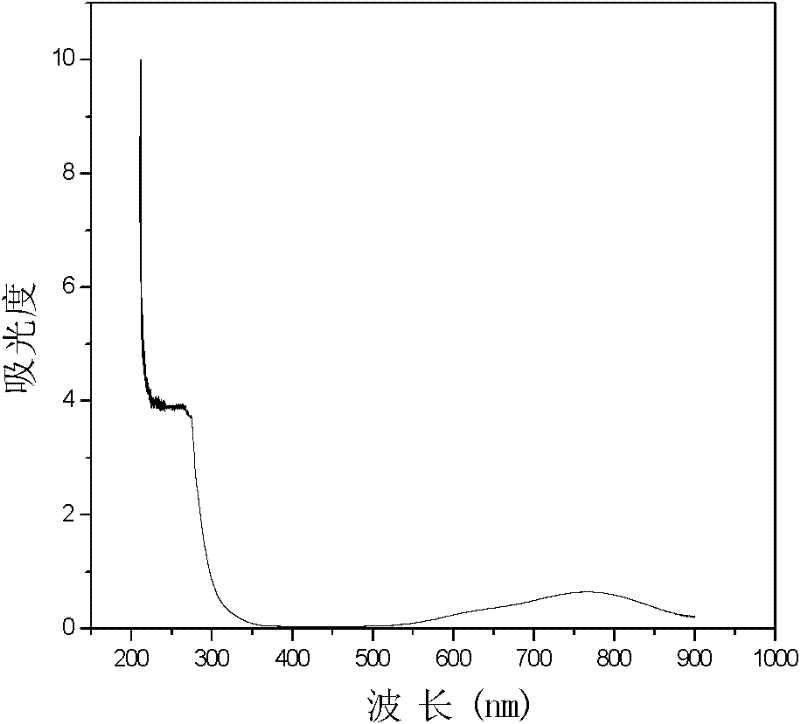
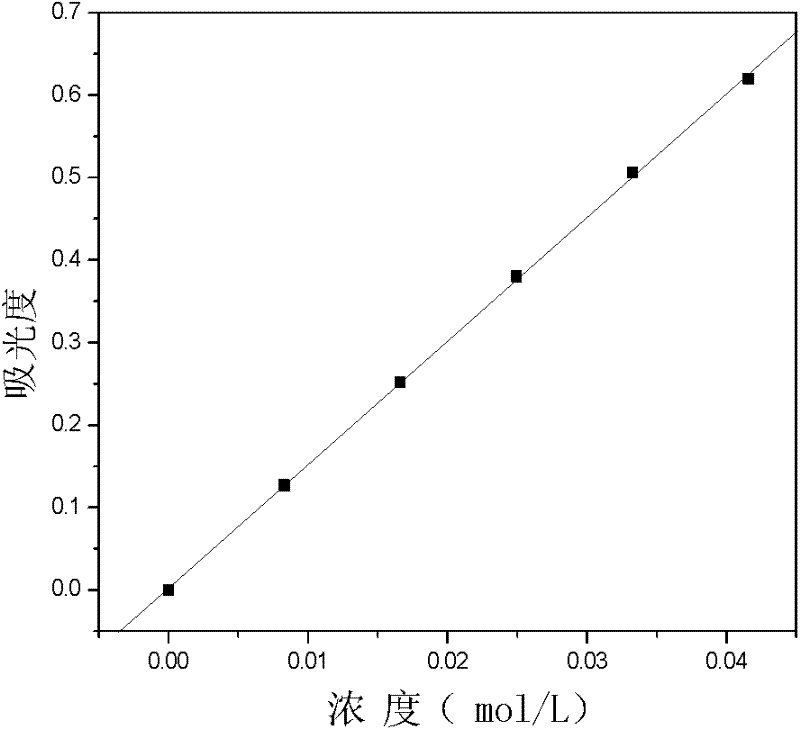
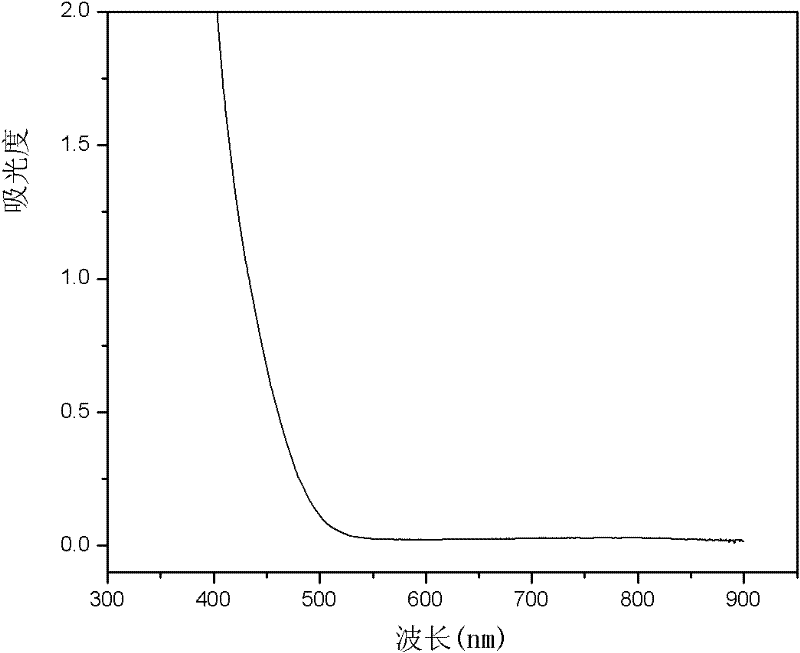
[0034] 3. The V(IV) standard sample is prepared into a V(IV) solution with a concentration of 0.02mol / L in an acidic solvent (acidic solvent is a sulfuric acid solution, and its concentration is 0.5mol / L), and the V(IV) solution is carried out full spectrum scan ( figure 1 ), determine ...
Embodiment 2
[0043] The ultraviolet quantitative determination method of the positive electrode electrolyte concentration of the vanadium battery of the present embodiment comprises the following steps:
[0044] 1. Determine the number of vanadyl sulfate crystal water by thermogravimetric method, and use it as V (IV) standard sample.
[0045] 2. In the wavelength range of 190nm to 900nm, scan the spectrum of the sulfuric acid solution, the concentration of the sulfuric acid solution is 0.5mol / L. It is determined that the sulfuric acid solution has no characteristic absorption peak in the wavelength range of 190nm to 900nm, which will not interfere with the determination of the absorbance of vanadium ions.
[0046] 3. The V(IV) standard sample is prepared into a V(IV) solution with a concentration of 0.03mol / L in an acidic solvent (acidic solvent is a sulfuric acid solution, and its concentration is 0.5mol / L), and the V(IV) solution is carried out The full-spectrum scan determined that the...
Embodiment 3
[0055] The ultraviolet quantitative determination method of the positive electrode electrolyte concentration of the vanadium battery of the present embodiment comprises the following steps:
[0056] 1. Determine the number of vanadyl sulfate crystal water by thermogravimetric method, and use it as V (IV) standard sample.
[0057] 2. In the wavelength range of 190nm to 900nm, scan the spectrum of the sulfuric acid solution, the concentration of the sulfuric acid solution is 0.2mol / L. It is determined that the sulfuric acid solution has no characteristic absorption peak in the wavelength range of 190nm to 900nm, which will not interfere with the determination of the absorbance of vanadium ions.
[0058] 3. The V(IV) standard sample is prepared into a V(IV) solution with a concentration of 0.05mol / L in an acidic solvent (acidic solvent is a sulfuric acid solution, and its concentration is 0.5mol / L), and the V(IV) solution is carried out The full-spectrum scan determined that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com