Use of hepatocyte nuclear factor-1alpha in treatment of chronic liver disease
A chronic liver disease, gene therapy technology, applied in genetic engineering and clinical medicine such as disease treatment, molecular biology, cell biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
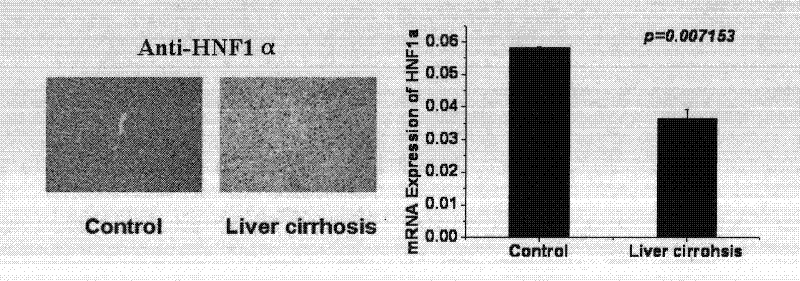
[0036] Differential expression of HNF1α protein and mRNA in normal human liver tissue and liver cirrhosis tissue detected by immunohistochemistry and Real-time PCR
[0037] 1 Immunohistochemical method was used to detect the changes of HNF1α protein in liver tissue.
[0038] Human normal liver tissue and liver cirrhosis tissue wax block 4mm serial section, baked in 60℃ oven for 30min and fixed, dewaxed to water, 3%H 2 o 2Place at room temperature for 10 minutes to remove endogenous peroxidase, and microwave citrate buffer for antigen retrieval, add 1:20 normal rabbit serum to block at room temperature for 30 minutes, add HNF1α antibody (1:200) dropwise at 4°C overnight; the next day PBS (0.01 M, pH 7.4) for 3 times, 5 min each time; add secondary antibody and incubate at room temperature for 30 min; after washing with PBS, add SABC (1:100) and incubate at room temperature for 20 min, develop color with DAB, mount with conventional resin, and observe under a light microscope. ...
Embodiment 2
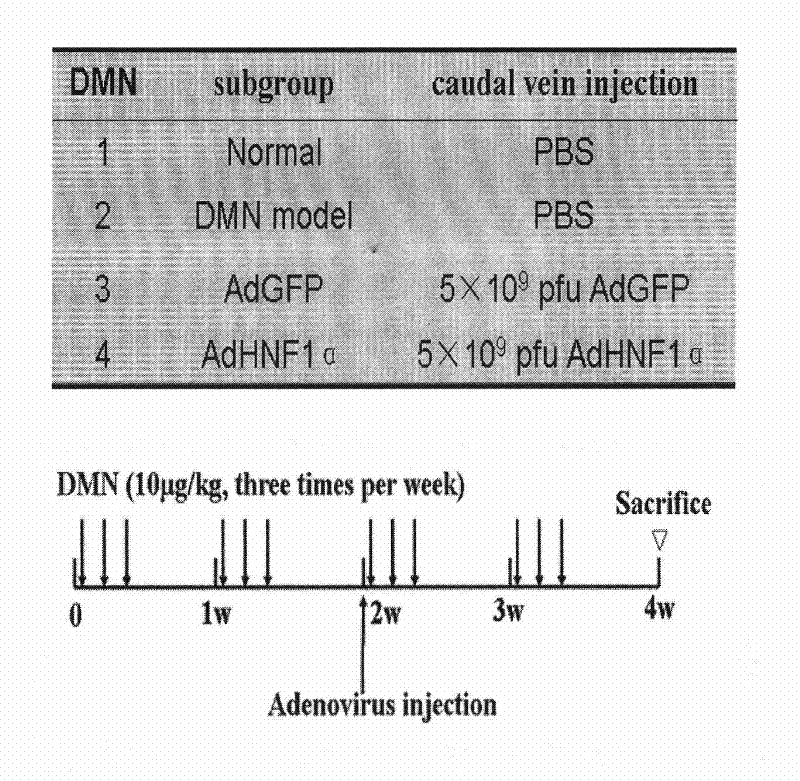
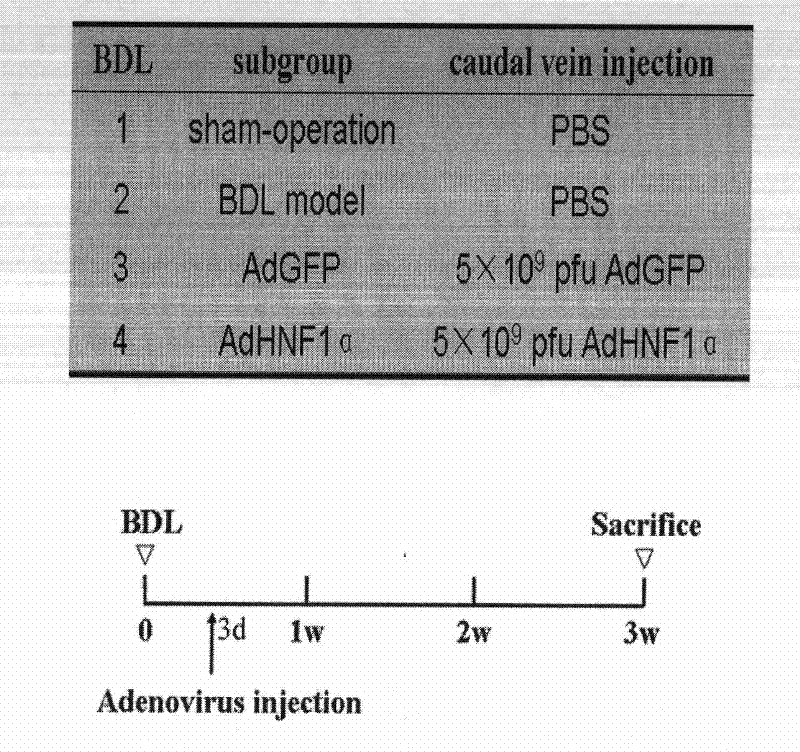
[0057] Inhibitory effect of up-regulated HNF1α expression on liver fibrosis progression in rats.
[0058] 1 Liver fibrosis model preparation:
[0059] DMN injury liver fibrosis model: Male SD rats were randomly divided into 4 groups, 10 in each group. Common food feeding, free drinking water, day and night lighting alternately. Group 1 received intraperitoneal injection of normal saline as the negative control group; Groups 2 to 4 were the liver fibrosis model group, and received intraperitoneal injection of 1% DMN solution at a dose of 10 μg / kg, three times a week for a total of 4 weeks. A rat liver fibrosis model induced by DMN was prepared. BDL liver fibrosis model: 40 male SD rats were divided into 4 groups, 10 in each group. Common food feeding, free drinking water, day and night lighting alternately. The first group is the sham operation group, and the second to fourth groups are the BDL group. After BDL rats were anesthetized by intraperitoneal injection of 10% chl...
Embodiment 3
[0066] Real-time PCR and immunohistochemical methods confirmed: HNF1α significantly inhibited HSC activation and extracellular matrix deposition in vivo, and blocked the EMT process of liver fibrosis.
[0067] 1 Extract total RNA from liver tissue of rats in each group of DMN and BDL liver injury models: 0.5-0.8g liver tissue, add TRizol reagent (1ml / 100mg tissue), crush the tissue until it is homogenized, and place it at room temperature for 5min; add chloroform 0.2 ml / ml TRizol, shake vigorously for 15s, then let stand at room temperature for 3min; Centrifuge at 12000rpm for 10min; discard the supernatant, add absolute ethanol 1ml / ml TRizol, and vortex to mix; centrifuge at 4°C, 7500rpm for 5min, discard ethanol, and dry naturally at room temperature; add 50μl DEPC water to dissolve the RNA; after the RNA is fully dissolved 1 μl each was subjected to 1.5% agarose gel electrophoresis, and the optical density values at 260 nm and 280 nm wavelengths were measured with a UV sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com