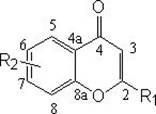
2-substituted chromone compound, as well as preparation method and application thereof
A kind of technology of chromone and compound, applied in the field of treatment or prevention of hepatitis B virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 extract compound
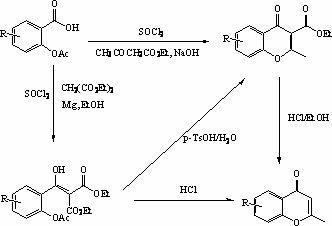
[0025] Oval Leaf Flower Anchor ( Halenia epliptica D. Don) (aerial part 5kg) was pulverized and infiltrated with 95% alcohol for 5 days. Concentrate under reduced pressure into alcohol extract, suspend in water and extract with petroleum ether and ethyl acetate respectively. After concentration of the water part, 95% alcohol was added for alcohol precipitation, and the precipitate was filtered off. The clear night was subjected to macroporous resin Diaion HP 20 column chromatography, and the water-ethanol gradient elution (0%-95%) was divided into five parts.
[0026] The part eluted with 20% alcohol was repeatedly eluted by gel Sephadex LH-20 column chromatography (water-methanol gradient elution, 0%-20%) and reverse phase C-18 silica gel column chromatography (30% methanol), get compound 1 (8-Hydroxy-2-methylchromone).
[0027] The part eluted with 95% alcohol was repeatedly eluted by gel Sephadex LH-20 column chromatography (water...
Embodiment 2
[0029] Example 2 extract compound
[0030] Oval Leaf Flower Anchor ( Halenia epliptica D. Don) (aerial part 5kg) was pulverized and infiltrated with 95% alcohol for 5 days. Concentrate under reduced pressure into alcoholic extract, suspend in water and extract with petroleum ether and ethyl acetate respectively. The ethyl acetate part was subjected to silica gel column chromatography (chloroform-acetone 1:0 gradient elution to 0:1).
[0031] Chloroform elution site was obtained by repeated silica gel column chromatography (chloroform elution) to obtain the compound 2 (8-methoxy-2-methylchromanone).
[0032] Chloroform-acetone (5:1) elution site was subjected to repeated silica gel column chromatography (chloroform-acetone 6:1 elution) to obtain the compound 1( 8-hydroxy-2-methylchromanone).
Embodiment 3
[0033] Example 3 In vitro anti-HBV activity test
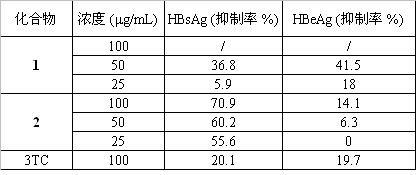
[0034] Apply Hep G 2 2.2.15 Cell lines, 10'10 per well 5 Cells were inoculated in a 96-well plate, the medium was DMEM, the growth medium contained 10% fetal bovine serum, 100 units / mL penicillin, 100 μg / mL streptomycin, 2 mmol / L L-glutamine, 5% CO 2 in the incubator37 o C culture, the compound medicine of the present invention is dissolved in dimethyl sulfoxide, is diluted to 100, 50 and 25 μ g / mL respectively, after cell culture 48 hours, changes into the culture medium containing medicine, continues to cultivate 9 days (changing medium every 2 days) Once), the supernatant was collected to detect HBsAg and HBeAg by ELISA method. Under the same conditions, the culture supernatant without drugs was used as a control, and lamivudine (3TC) was used as a positive control. The cytotoxicity of drugs was determined by MTT method. The results confirmed that 8-hydroxy-2-methylchromanone ( 1 ) and 8-methoxy-2-methyl ( 2 ) has...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com