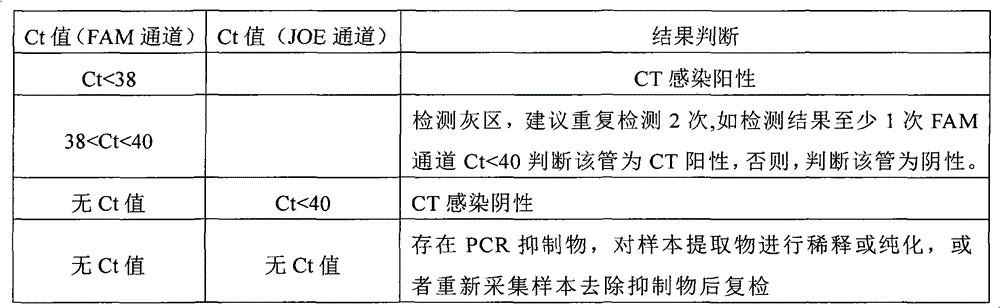
Chlamydia trachomatis nucleic acid rapid detection kit
A technology of Chlamydia trachomatis and a kit, which is applied in the field of biochemistry, can solve the problems of false negative results of PCR inhibitor detection, and achieve the effects of ensuring detection specificity, simple and rapid operation, and avoiding misdiagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of Chlamydia trachomatis nucleic acid rapid detection kit
[0046] The components in the kit are self-prepared, and the preparation process of each component of the 32-person kit is as follows:
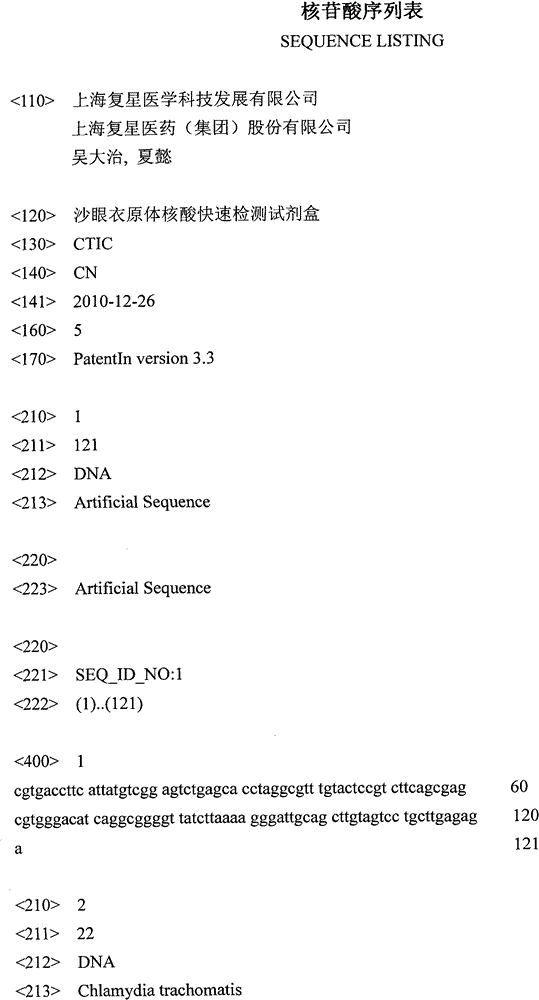
[0047] (1) CT PCR buffer 672μl, containing Tris-HCl (pH8.3) 14.2mM, KCl 71.4mM, gelatin 0.14mg / ml, dATP, dGTP, dCTP, dUTP each 0.43mM, MgCl 2 5 mM, 5 μM each of upstream and downstream primers (SEQ ID No: 2 and 3).
[0048] (2) 96 μl of fluorescent probe, containing 1.5 μM each of CT fluorescent probe (SEQ ID No: 4) and internal reference fluorescent probe (SEQ ID No: 5).
[0049] (3) 64 μl of Taq enzyme, containing 40 U of Taq DNA polymerase and 16 U of uracil-DNA glycosylase (UNG enzyme).
[0050] (4) Internal reference DNA 160μl, containing 10 5 copy / ml internal reference DNA (SEQ ID NO: 1).
[0051] (5) Negative control 200μl, containing 10 4 bacteria / ml E. coli culture.
[0052] (6) Positive control 200μl, containing 10 3 inclusion bodi...
Embodiment 2
[0055] Example 2: Application of the kit in the identification of Chlamydia trachomatis in male urethral swab samples
[0056] (1) Sample processing
[0057] Select 7 cases of male urethral swab samples whose two channels were negative for the initial detection of this reagent, and dilute the extract obtained by the sample processing method in the summary of the invention 5 times with DNA extract for amplification detection.
[0058] (2) Amplification detection
[0059] According to the method in the summary of the invention, take the kit components CT PCR buffer 21μl×9, fluorescent probe 3μl×9, Taq enzyme 2μl×9 and put them in a 1.5ml centrifuge tube, mix well and centrifuge briefly, each amplification tube Aliquot 26 μl, and add 4 μl each of the negative control and positive control extracts of the kit treated in the same way, and the above-mentioned 7 sample templates that were diluted. The volume of each reaction amplification tube is 30 μl. Put the above-mentioned ampli...
Embodiment 3
[0062] Example 3: Application of the kit in the identification of Chlamydia trachomatis in female cervical swabs
[0063] (1) Sample processing
[0064] Select 4 cases of female cervical swab samples whose two channels were negative in the initial detection of this reagent, take 200 μl of swab cleaning solution again, use QIAamp DNA mini kit from QIAGEN Company to process according to its instructions, and perform PCR detection.
[0065] (2) Amplification detection
[0066] According to the method in the summary of the invention, take the kit components CT PCR buffer 21μl×6, fluorescent probe 3μl×6, Taq enzyme 2μl×6 and put them in a 1.5ml centrifuge tube, mix them and centrifuge briefly, each amplification tube Aliquot 26 μl, and add 4 μl each of the negative control and positive control extracts of the kit treated according to the same method, and the above-mentioned 4 sample templates processed by the QIAGEN column method extraction kit. The volume of each reaction amplif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com