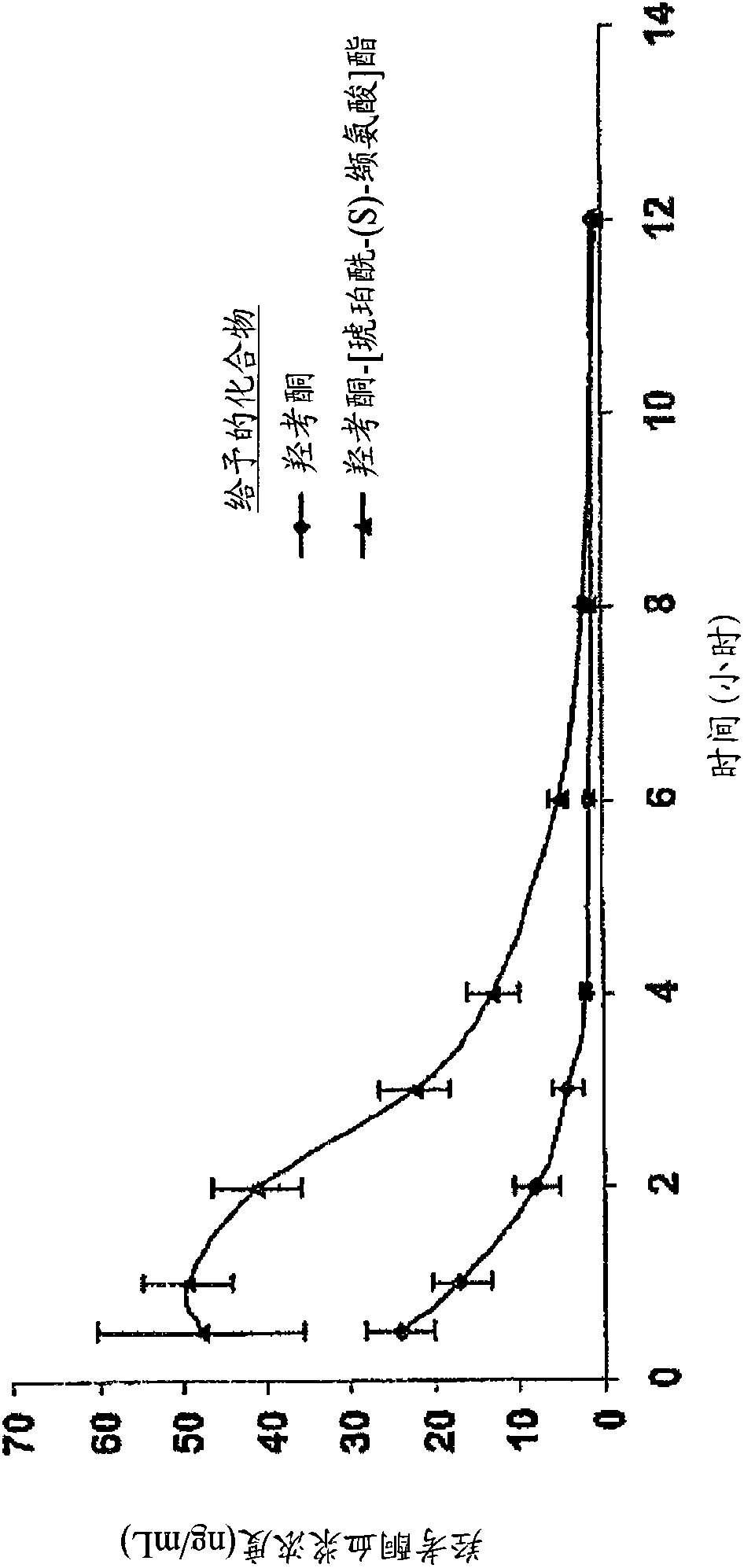
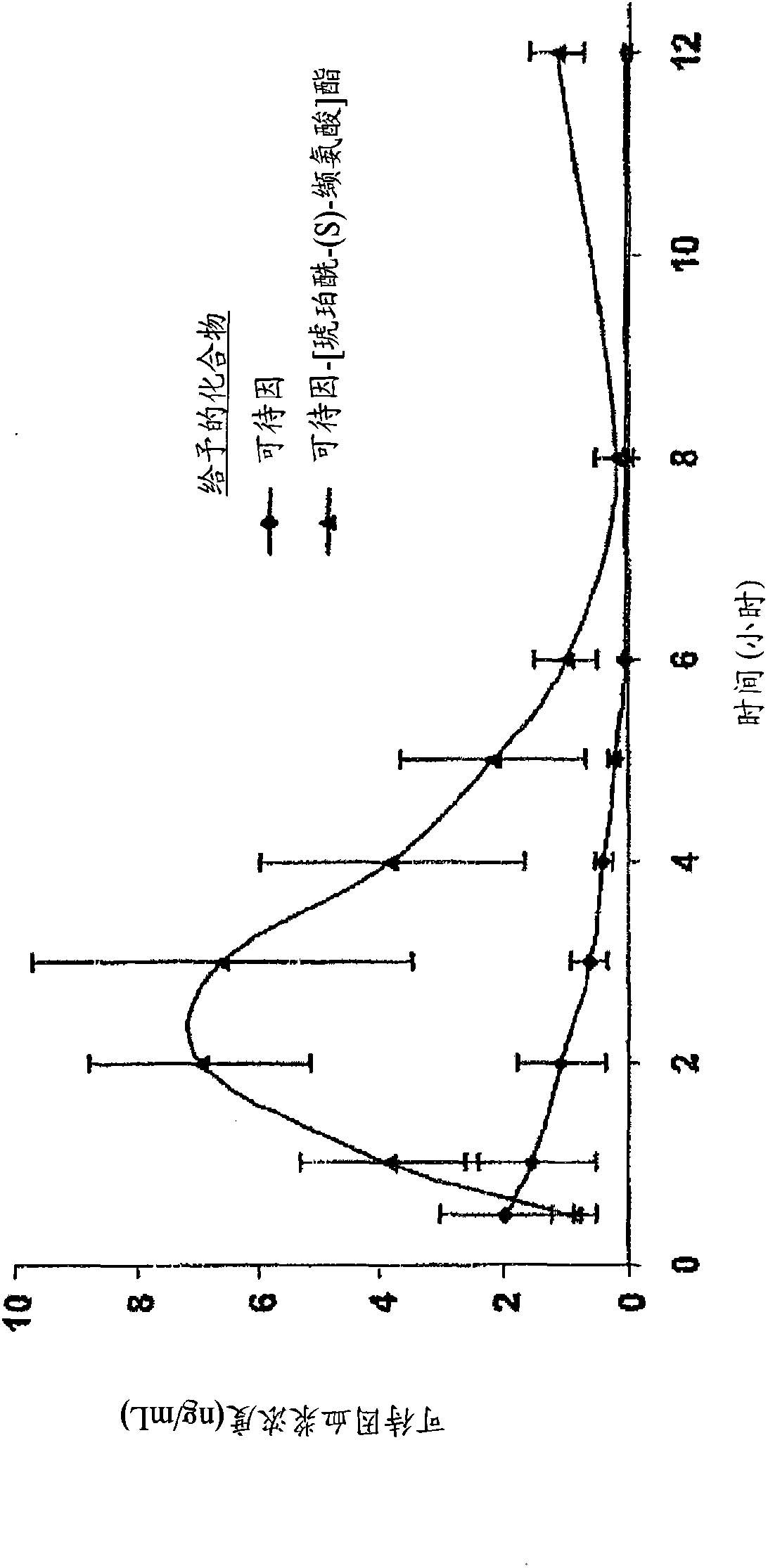
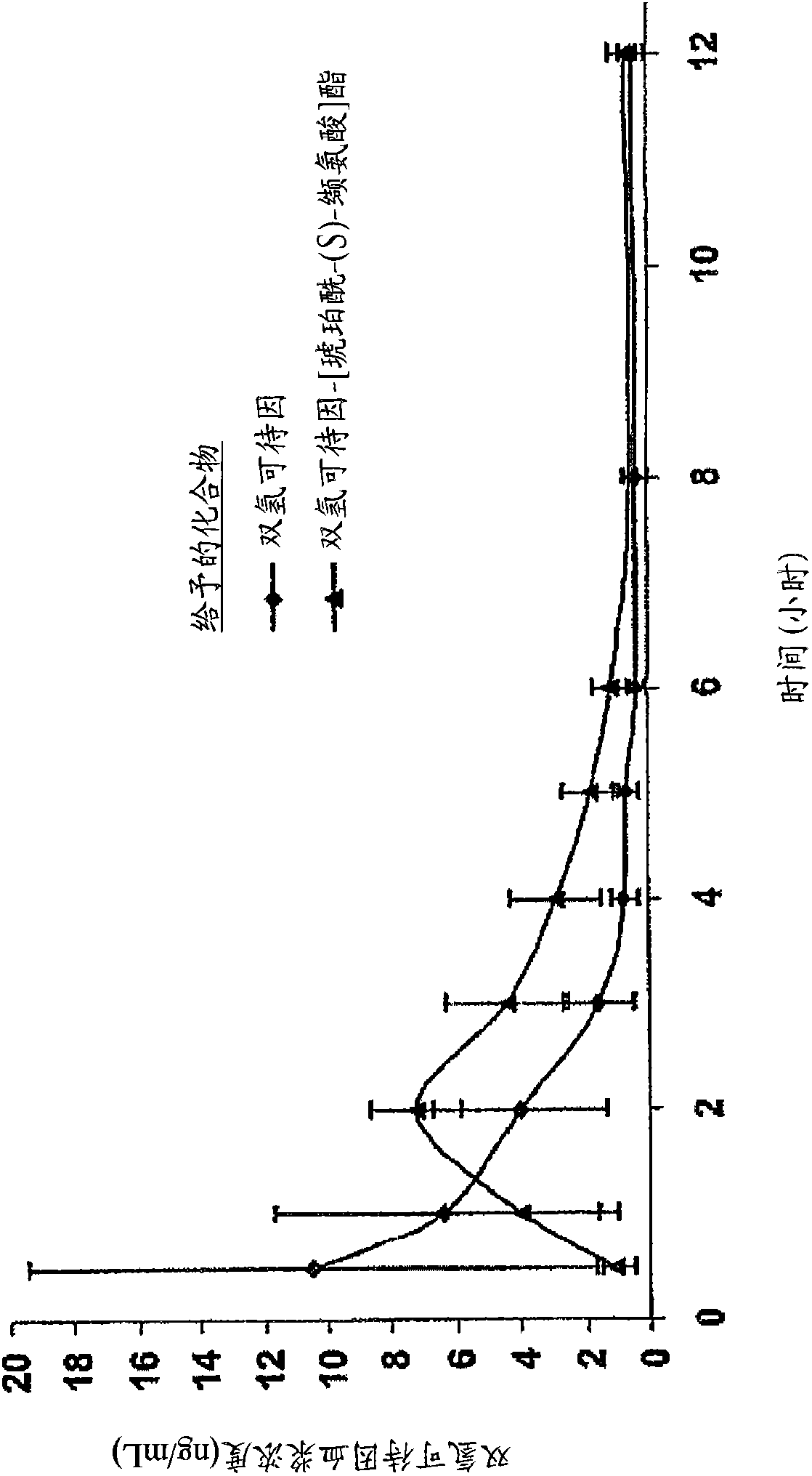
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
A technology of opioids and prodrugs, applied in the field of amino acid and peptide prodrugs, can solve problems such as unstable systemic utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] Conventional techniques for the preparation / isolation of individual enantiomers, if necessary, include chiral synthesis from suitable optically pure precursors, or resolution of racemates (or salts) using, for example, chiral high pressure liquid chromatography (HPLC). or racemates of derivatives).
[0115] Alternatively, the racemate (or racemic precursor) can be reacted with a suitable optically active compound, such as an alcohol, or in case the compound of formula (I) contains an acidic or basic moiety, a base or acid , such as 1-phenylethylamine or tartaric acid. The resulting diastereomeric mixtures can be separated by chromatography and / or fractional crystallization and one or both diastereoisomers converted into the corresponding pure enantiomers by methods well known to the skilled person.
[0116] The chiral compounds of the present invention (and their chiral precursors) can be obtained in enantiomerically enriched form by chromatography, usually HPLC, on an...
Embodiment 1
[0787] Example 1 - General route to the synthesis of amino acid or peptide dicarboxylic acid conjugates of opioids
[0788] Schemes 1 (alcohol esters) and 2 (enol esters) below give two general routes for the synthesis of dicarboxylic acid-linked amino acid or peptide conjugates of opioids as their HCl or TFA salts. These synthetic routes are illustrated using a succinic acid linker. However, this applies to all dicarboxylic acid linkers of the invention.
[0789]
[0790] Scheme 1 - Route of dicarboxylic acid-linked amino acid opioid prodrugs (alcohol esters)
[0791]
[0792] Scheme 2 - Route of dicarboxylic acid-linked amino acid opioid prodrugs (enol esters)
[0793] The compounds listed in Table 8 can be prepared by these methods using meprotamol and valine as examples of hydroxy opioids and amino acids, respectively. It should be understood that other opioids can readily be substituted for meprotamol for conjugation to the various prodrug moieties described he...
Embodiment 2-
[0803] The synthesis of embodiment 2-oxycodone-[succinyl-(S)-valine] enol ester
[0804] Scheme 3 gives the general synthetic route of oxycodone-[succinyl-(S)-valine] ester.
[0805]
[0806] Scheme 3 - Synthesis of oxycodone-[succinyl-(S)-valine] enol ester from activated succinyl-(S)-valine ester
[0807] Synthesis of (N-hydroxysuccinimide)-succinyl-(S)-valine-O-tert-butyl ester in detail described
[0808] A solution of N,N-dicyclohexylcarbodiimide (958 mg, 4.64 mmol) in ethyl acetate (15 mL) was added to succinyl-(S)-valine- O-tert-butyl ester (1.21 g, 4.42 mmol) and N-hydroxysuccinimide (560 mg, 4.86 mmol). The reaction was stirred at 50 °C for 2 hours. The resulting mixture was cooled to room temperature and filtered through celite. The filtrate was washed twice with saturated aqueous sodium bicarbonate (50 mL), water (50 mL) and brine (50 mL), dried (MgSO 4 ) and concentrated to give the desired (N-hydroxysuccinimide)-succinyl-(S)-valine-O-tert-butyl ester...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com