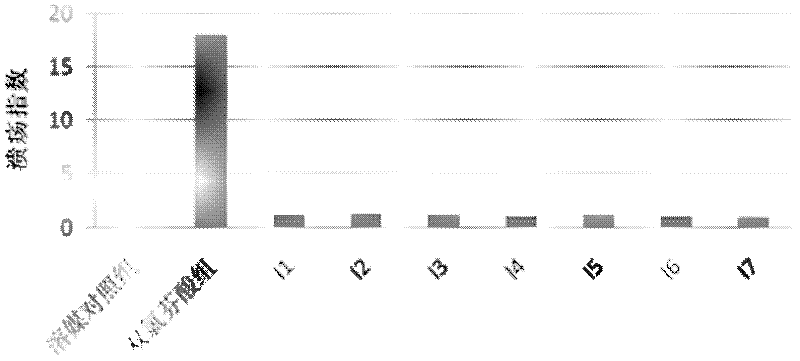
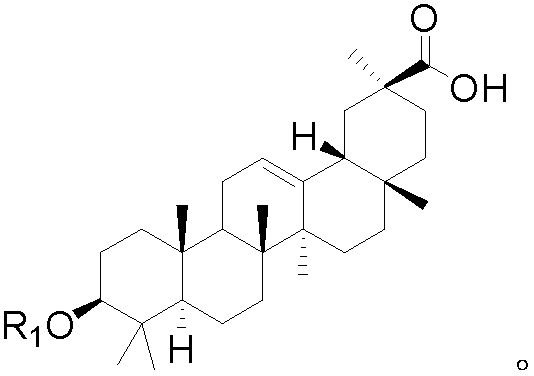
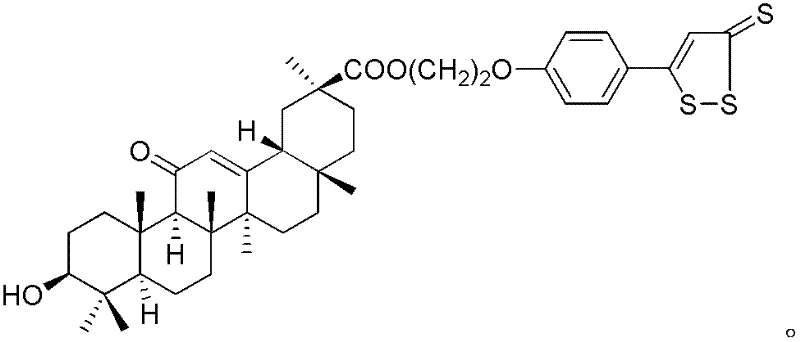
Application of glycyrrhetinic acid derivatives in preparation process of anti-inflammatory drugs
A technology of glycyrrhetinic acid and anti-inflammatory drugs, applied in the direction of anti-inflammatory agents, drug combinations, antipyretics, etc., can solve the problem of no anti-inflammatory activity, etc., to avoid gastrointestinal damage and/or cardiovascular adverse events or other toxic side effects, reducing cardiovascular events, high anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 5-[4-(2-bromoethoxy)phenyl]-3H-1,2-dithiol-3-thione (5a)
[0037] ADT-OH (0.325g, 1.4mmol), 1,2-dibromoethane (0.50mL, 5.8mmol), anhydrous K 2 C0 3 (0.396g, 2.8mmol), dissolved in 10mL dry DMF, reacted at 120°C for 2h. After cooling, add 20mL ethyl acetate to dilute, wash with water (3×20mL), anhydrous Na 2 SO 4 dry. Filter, evaporate to dryness under reduced pressure, and recrystallize from acetone-water to obtain 0.388 g of dark brown product with a yield of 81.5%, mp: 126.0-127.0°C. 1 HNMR(400MHz, CDCl 3 ), δ (ppm): 7.59 (d, 2H, J = 8.9 Hz, ArH), 7.36 (s, 1H, = CH), 6.96 (d, 2H, J = 8.9 Hz, ArH), 4.33 (t, 2H , J=6.1Hz, CH 2 ), 3.65(t, 2H, J=6.1Hz, CH 2 ); 13 CNMR(400MHz, CDCl 3 ), δ (ppm): 212.554, 170.180, 158.775, 132.260, 126.120, 122.226, 113.014, 65.455, 26.026.
[0038] The above identification data proves that the compound obtained is 5-[4-(2-bromoethoxy)phenyl]-3H-1,2-dithiol-3-thione (5a), and its structural formula is:
[0039] Compound I 1 Prepa...
Embodiment 2
[0044] Preparation of 5-[4-(3-bromopropoxy)phenyl]-3H-1,2-dithiol-3-thione (5b)
[0045] Using 1,3-dibromopropane as the raw material, it was prepared by referring to the method of 5a, the yield was 83.2%, mp: 79.0-80.0°C. 1 HNMR(400MHz, CDCl 3 ), δ (ppm): 7.62 (d, 2H, J = 8.8 Hz, ArH), 7.40 (s, 1H, = CH), 7.00 (d, 2H, J = 8.7 Hz, ArH), 4.19 (t, 2H , J=5.8Hz, CH 2 ), 3.62(t, 2H, J=6.3Hz, CH 2 ), 2.36(p, 2H, J=6.0Hz, CH 2 ); 13 CNMR(400MHz, CDCl 3 ), δ (ppm): 212.460, 170.437, 159.454, 132.073, 126.065, 121.751, 112.886, 63.118, 29.453, 27.178.
[0046] The above identification data proves that the obtained compound is 5-[4-(3-bromopropoxy)phenyl]-3H-1,2-dithio-3-thione (5b), and its structural formula is:
[0047] Compound I 2 Preparation
[0048] Using GA and 5b as raw materials, refer to I 1 Prepared by synthetic method, red solid, yield 86.6%, mp: 79.1-80.7°C. 1 HNMR(400MHz, CDCl 3 ), δ (ppm): 7.54 (d, 2H, J = 8.8 Hz, ArH), 7.31 (s, 1H, = CH), 6.91 (d, 2H, J = 8.8 Hz, ArH), 5.54 (...
Embodiment 3
[0052] Preparation of 5-[4-(4-bromobutoxy)phenyl]-3H-1,2-dithiol-3-thione (5c)
[0053] Using 1,4-dibromobutane as the raw material, it is prepared by referring to the method of 5a, the yield is 79.4%, mp: 70.0-71.0°C. 1 HNMR(400MHz, CDCl 3 ), δ (ppm): 7.60 (d, 2H, J = 8.7 Hz, ArH), 7.38 (s, 1H, = CH), 6.96 (d, 2H, J = 8.7 Hz, ArH), 4.07 (t, 2H , J=5.9Hz, CH 2 ), 3.50(t, 2H, J=6.4Hz, CH 2 ), 2.08(m, 2H, CH 2 ), 1.99(m, 2H, CH 2 ); 13 CNMR(400MHz, CDCl 3 ), δ (ppm): 212.439, 170.554, 159.670, 132.006, 126.057, 121.553, 112.834, 64.759, 30.795, 26.729, 25.133.
[0054] The above identification data proves that the compound obtained is 5-[4-(4-bromobutoxy)phenyl]-3H-1,2-dithiol-3-thione (5c), and its structural formula is:
[0055] Compound I 3 Preparation
[0056] Using GA and 5c as raw materials, refer to I 1 Prepared by synthetic method, red solid, yield 89.2%, mp: 138.2-139.2°C. 1 HNMR(400MHz, CDCl 3 ), δ (ppm): 7.60 (d, 2H, J = 8.8 Hz,), 7.39 (s, 1H, = CH), 6.98 (d, 2H, J = 8.8 Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com