Engineering bacteria for generating gentamicin C1a and constructing method of engineering bacteria
A technology of gentamicin and its construction method, applied in the field of engineering bacteria producing gentamicin C1a and its construction, can solve the problems of unclear genetic background research of bacterial strains, etc., achieve clear genetic background of bacterial strains, increase yield, and quickly screen Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
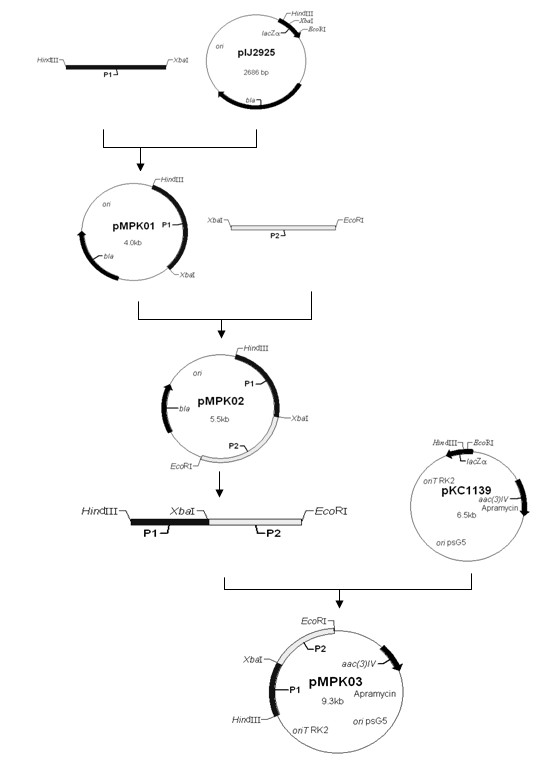
[0034] Example 1: gntK Construction of gene blocking plasmid
[0035] According to the published Micromonospora echinospora Design primers with corresponding sequence (GenBank accession number: AY524043):
[0036] Upstream primer of the left arm: K1 5’ -TCCAAGCTTGTACCCTCGGAGCCGGTCTTGT-3’ with Hin dⅢ restriction site;
[0037] Downstream primer of the left arm: K2 5’ –TGCTCTAGACTGGGTTGCGTCTGCGTGAT -3’ with Xba Ⅰ Restriction site; Amplify 1.3kb fragment P1;
[0038] Right arm upstream primer: K3 5’ -TGCTCTAGACGGGTAGAACGGGTTGGTCC -3’ with Xba Ⅰ restriction site;
[0039] Downstream primer of right arm: K4 5’ -CGGAATTCCAGCGTTGGCAATAATCATCAGC -3’ with Eco RⅠ restriction site; Amplify 1.5kb fragment P2.
[0040] Using the total DNA of Micromonospora purpurea as a template, the two fragments 1.3kb and 1.5kb were amplified by PCR, and the conservative sequence of 348bp within the gene was deleted. The conditions of the PCR reaction were: (1) 95℃ pre-denaturation for 5 minutes; ( 2) Melti...
Embodiment 2
[0041] Example 2: Blocking plasmid pMPK03 to transform Micromonospora crassa
[0042] Will contain ori The shuttle plasmid of T was transformed into E. coli ET12567 (pUZ8002), and the donor strain E. coli ET12567 (pUZ8002, donor plasmid) was obtained. A single colony of E. coli ET12567 (pUZ8002, donor plasmid) was inoculated in 3 ml of LB medium (adding action concentrations of 25 μg / ml kanamycin and chloramphenicol and 50 μg / ml donor plasmid corresponding antibiotics) Incubate overnight at 37°C, transfer 0.2% to 20 ml of LB medium containing three antibiotics, incubate at 37°C for 2.5~3.0 h, centrifuge to pellet the bacteria, wash twice with fresh LB medium, and finally The bacteria are suspended in about 7.5 ml of medium, and the microscopic examination count is about 10 8 Magnitude.
[0043] Prepare the single spore suspension of Micromonospora cristatum, wash it twice with 2×YT medium and dissolve it in a certain volume of 2×YT to make the concentration of single spore 10 8 ,...
Embodiment 3
[0045] Example 3: Screening of double exchange blocking strains
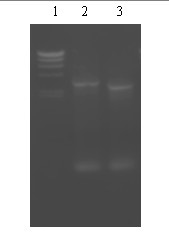
[0046] The transformants grown at 28°C were cultured at 37°C. Because the blocking plasmid cannot replicate at 42°C, the zygote will grow only after it is integrated into the chromosome through homologous exchange. Antibiotic resistance indicates that the zygote has undergone single exchange integration. The single exchange strains were uploaded to the drug-free plate for three generations, and 350 single colonies were picked and planted on the drug-containing and drug-free plates respectively. Two apramycin-sensitive strains were selected and the total DNA of the two strains was extracted. PCR verified that one of the strains was as big as the control, indicating that it had a single exchange and shedding; the other strain had a smaller fragment than the control, indicating that it had a double exchange. This strain was named M.purpurea gntK - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com