Chemical method for preparing cobalt nickel nanoscale alloy powder
A technology of nano-alloy powder and cobalt-nickel, which is applied in the field of preparation of cobalt-nickel nano-alloy powder, can solve the problems of complex experimental process, long incubation time, and difficult control of product components, and achieve mild reaction conditions, simple process, and easy preparation low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of reaction solution:
[0030] 1) Preparation of metal salt solution:
[0031] Use ethylene glycol as solvent to prepare solutions in the following proportions.
[0032]
[0033]
[0034] 2) Preparation of reducing agent solution:
[0035] Use ethylene glycol as solvent to prepare solutions in the following proportions.
[0036]
[0037] 2. Cobalt nanowire preparation:
[0038] ① Heat the solutions prepared by the above method to 90°C respectively;
[0039] ②Add the reducing agent solution dropwise into the metal salt solution;
[0040] ③React for 20 minutes until the reaction is complete;
[0041] ④Centrifugal separation solution and cobalt-nickel nano-alloy powder;
[0042] ⑤ Wash the cobalt-nickel nano-alloy powder 3 times with deionized water, acetone, and absolute ethanol successively;
[0043] ⑥ dry collection.
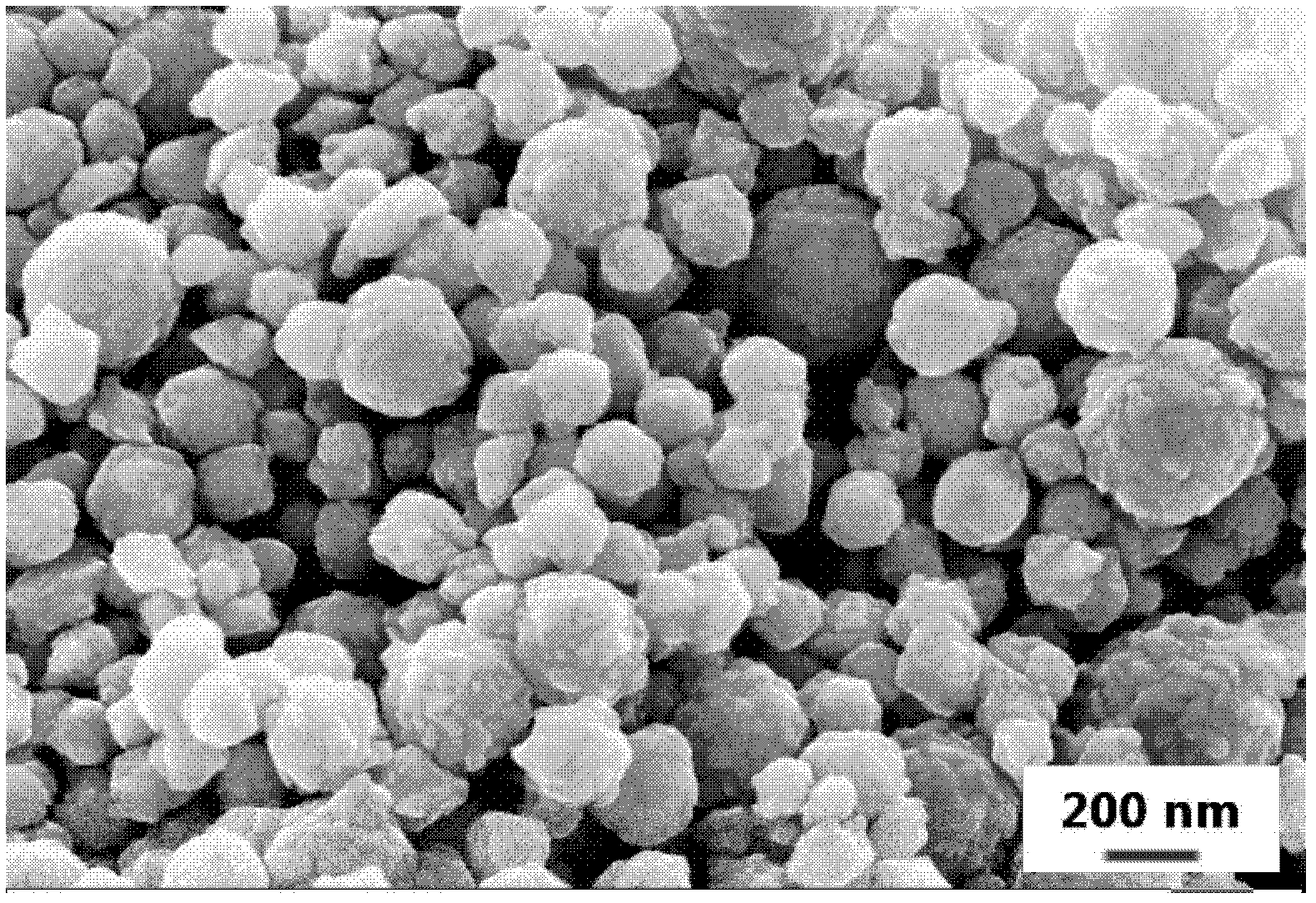
[0044] The obtained powder is spherical, uniformly distributed, and the average particle size is 187 nm. It looks like ...
Embodiment 2
[0046] 1. Preparation of reaction solution:
[0047] 1) Preparation of metal salt solution:
[0048] Prepare solutions in the following ratios using deionized water as solvent.
[0049]
[0050] 2) Preparation of reducing agent solution:
[0051] Prepare solutions in the following ratios using deionized water as solvent.
[0052]
[0053] 2. Cobalt nanowire preparation:
[0054] ① Heat the solutions prepared by the above method to 90°C respectively;
[0055] ②Add the reducing agent solution dropwise into the metal salt solution;
[0056] ③React for 20 minutes until the reaction is complete;
[0057] ④Centrifugal separation solution and cobalt-nickel nano-alloy powder;
[0058] ⑤ Wash the cobalt-nickel nano-alloy powder 3 times with deionized water, acetone, and absolute ethanol successively;
[0059] ⑥ dry collection.
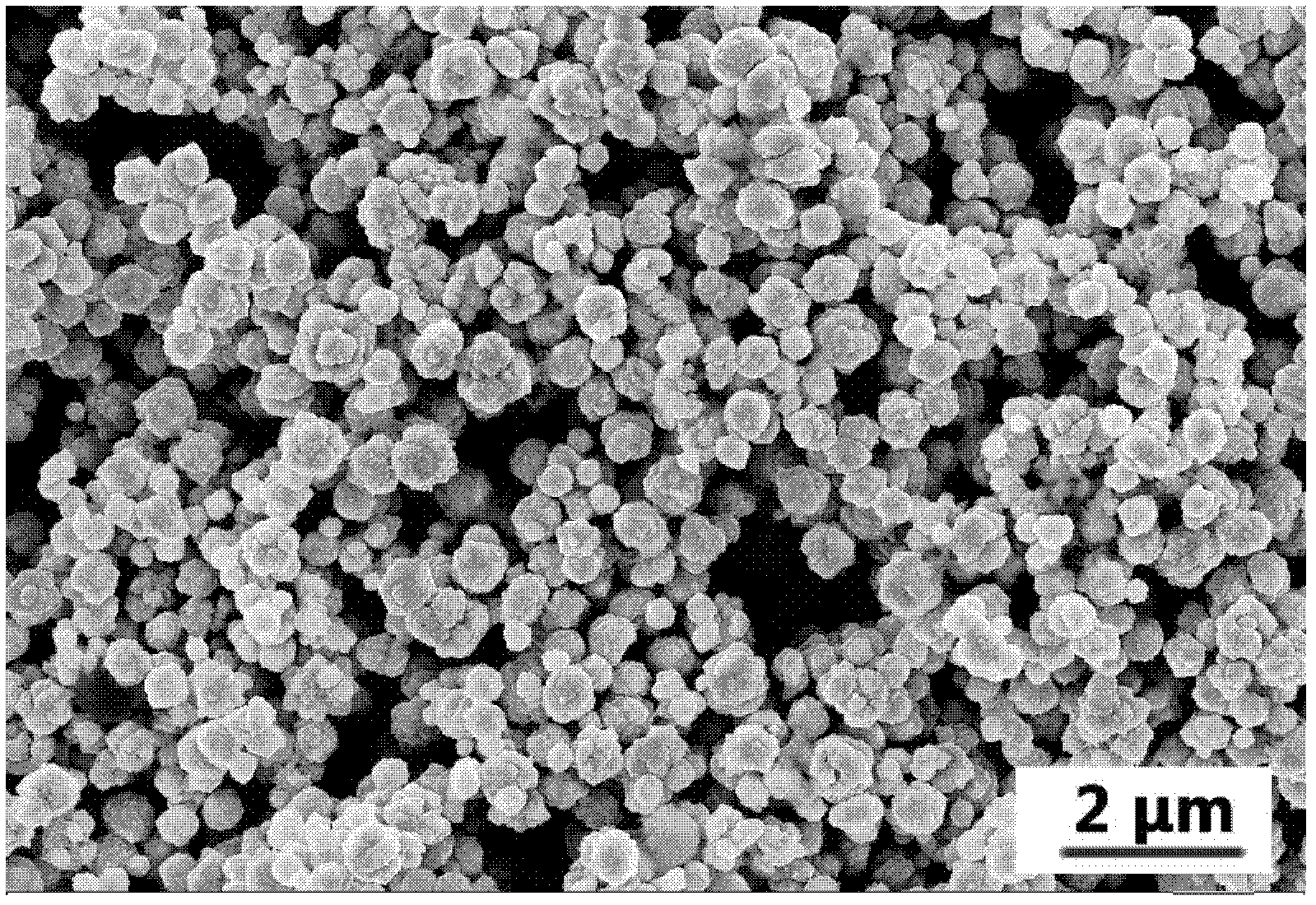
[0060] The obtained powder is spherical, uniformly distributed, and the average particle size is 380 nm. It looks like figure 2 shown.
Embodiment 3
[0062] 1. Preparation of reaction solution:
[0063] 1) Preparation of metal salt solution:
[0064] Use ethylene glycol as solvent to prepare solutions in the following proportions.
[0065]
[0066] 2) Preparation of reducing agent solution:
[0067] Use ethylene glycol as solvent to prepare solutions in the following proportions.
[0068]
[0069] 2. Cobalt nanowire preparation:
[0070] ① Heat the solutions prepared by the above method to 90°C respectively;
[0071] ②Add the reducing agent solution dropwise into the metal salt solution;
[0072] ③React for 20 minutes until the reaction is complete;
[0073] ④Centrifugal separation solution and cobalt-nickel nano-alloy powder;
[0074] ⑤ Wash the cobalt-nickel nano-alloy powder 3 times with deionized water, acetone, and absolute ethanol successively;
[0075] ⑥ dry collection.
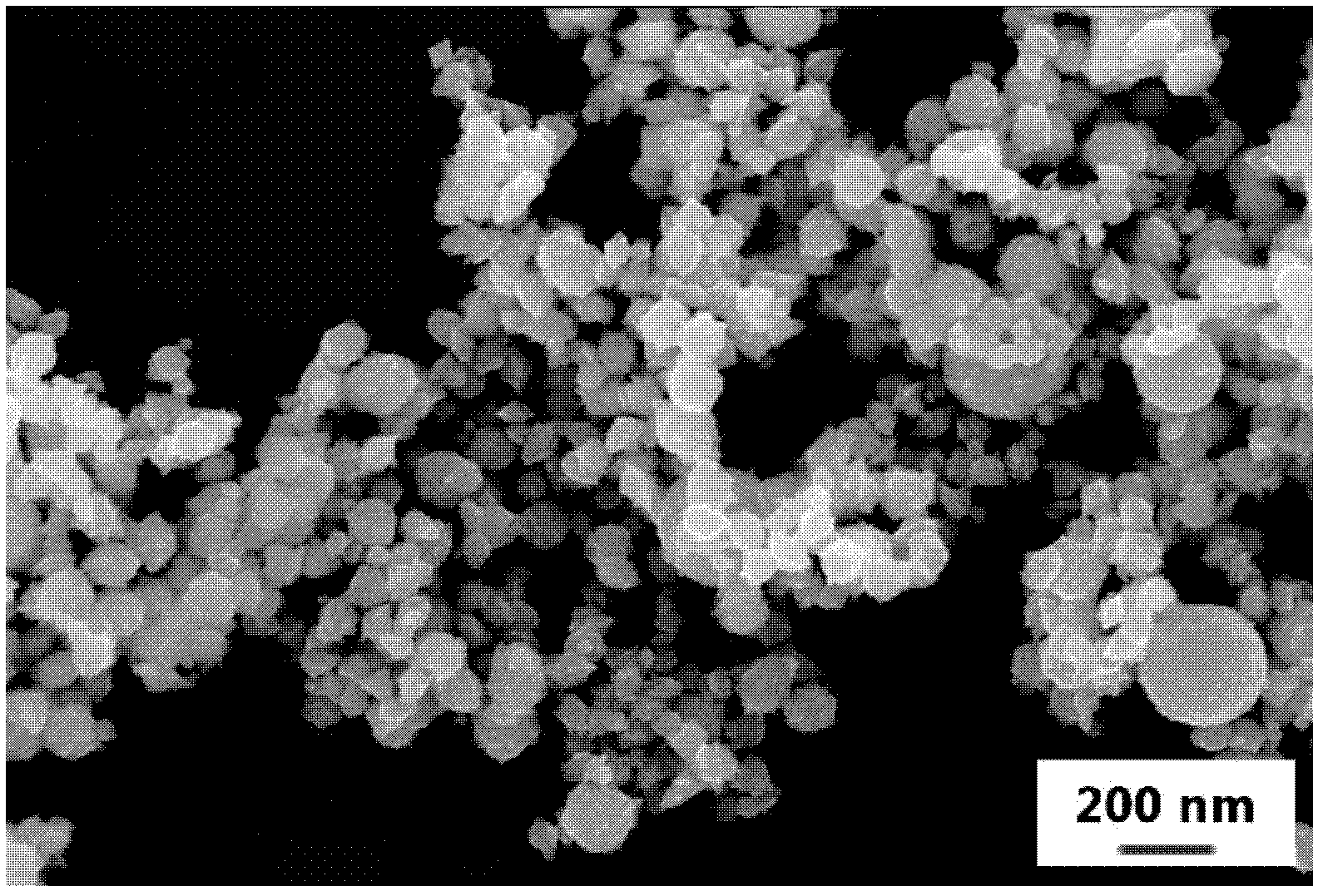
[0076] The obtained powder is spherical, uniformly distributed, and the average particle size is 95nm. It looks like image 3 shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com