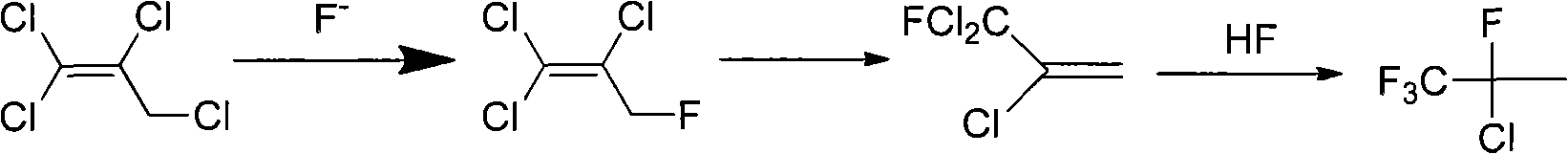Preparation method of 2-chloro-1,1,1,2-tetrafluoropropane
A technology of tetrafluoropropane and fluoropropene, which is applied in the field of 2-chloro-1, can solve the problems of difficult separation and high temperature of gas-phase fluorination reaction, and achieve the effect of saving energy and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1,1,2,3-Tetrachloropropene undergoes a nucleophilic substitution reaction with a nucleophilic fluorinating reagent to obtain 1,1,2-trichloro-3-fluoropropene, and the nucleophilic fluorinating reagent is potassium fluoride.
[0022] Add 1,1,2,3-tetrachloropropene (5.0g, 0.028mol) and 30mL of diethylene glycol successively into a 50mL dry there-necked flask equipped with a magnetic stirrer, a thermometer, and a condensing device, and heat up under constant stirring To 120°C, add potassium fluoride (4.87g, 0.084mol) under constant stirring, the molar ratio of potassium fluoride to 1,1,2,3-tetrachloropropene is 3, and react at 120°C for 2h . The reaction product was analyzed by gas chromatography, and the results showed that the conversion rate of 1,1,2,3-tetrachloropropene was 98%, and the selectivity of 1,1,2-trichloro-3-fluoropropene was 90%.
Embodiment 2~6
[0024] The operating process of Examples 2-6 is similar to that of Example 1, except that the nucleophilic fluorinating reagents used are different, and the reaction results are shown in Table 1.
[0025] Table 1
[0026] Example Nucleophilic fluorinating reagents Conversion rates(%) selectivity (%) 2 sodium fluoride 54 85 3 cesium fluoride 99 92 4 Potassium bifluoride 85 95 5 Potassium bifluoride 45 98 6 cesium hydrogen fluoride 90 94
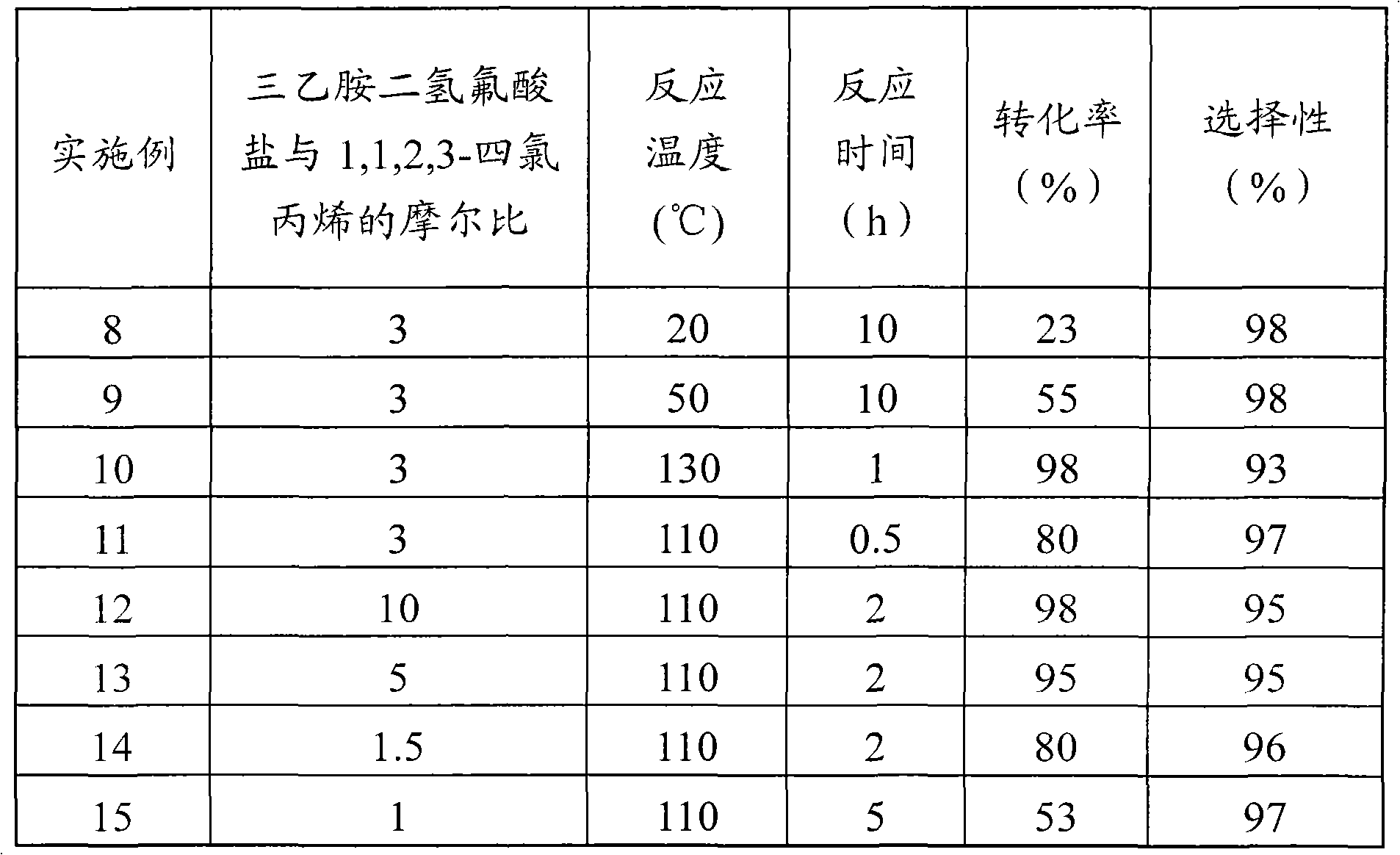
Embodiment 7
[0028] 1,1,2,3-Tetrachloropropene undergoes a nucleophilic substitution reaction with a nucleophilic fluorinating reagent to obtain 1,1,2-trichloro-3-fluoropropene, and the nucleophilic fluorinating reagent is triethylamine dihydrofluoric acid Salt.
[0029] Add the nucleophilic fluorinating agent triethylamine dihydrofluoride (NEt 3 2HF) (10.8g, 0.084mol), then add 1,1,2,3-tetrachloropropene (5.0g, 0.028mol), triethylamine dihydrofluoride and 1,1,2,3-tetrachloropropene The molar ratio of allyl chloride was 3, and the reaction was carried out at a reaction temperature of 110°C for 2 hours. The reaction product was analyzed by gas chromatography, and the results showed that the conversion rate of 1,1,2,3-tetrachloropropene was 90%, and the selectivity of 1,1,2-trichloro-3-fluoropropene was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com