Derivatives of anti-influenza and anti-avian influenza medicament and application thereof
A technology of derivatives and uses, applied in the field of pharmaceutical compounds, can solve the problems of low oral bioavailability and difficulty in permeating small intestinal epithelial cells after oral administration
- Summary
- Abstract
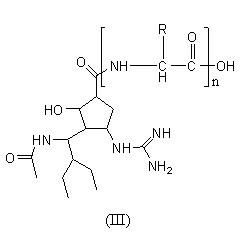
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
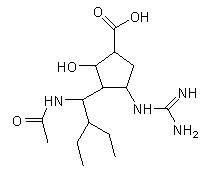
[0031] Example 1: (1S,2S,3R,4R)-3-[(1S)-1-acetamido-2-ethyl-butyl]-4-guanidino-2-hydroxy-cyclopentyl-1 - Carboxylic acid L-valine ester (compound 1-1)
[0032]
[0033] Preparation Process:
[0034] The first step: 3285 mg (10 mmol) of compound A, 10.9 g of di-tert-butyl dicarbonate and 8340 mg of potassium carbonate were added to 100 ml of ethyl acetate solution. After stirring at room temperature for 12 hours, add 100 ml of water until the solid is completely dissolved, collect the organic layer, then wash the organic layer with water, dry over anhydrous sodium sulfate, and distill off the solvent to obtain a colorless oil.
[0035] Step 2: Add carbonyldiimidazole (5 mmol) to tetrahydrofuran, then add 2640 mg (5 mmol) of the oil obtained in the first step, stir at 25 °C for 1.5 hours, then stir at 45 °C 1 hour. Under stirring conditions, L-valine in anhydrous dimethylformamide (DMF) solution was reacted at 80° C. for 2 hours in the above solution. Evaporate DMF under...
Embodiment 2
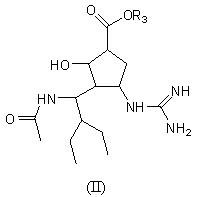
[0037] Example 2 (1S, 2S, 3R, 4R)-3-[(1S)-1-acetamido-2-ethyl-butyl]-4-guanidino-2-hydroxyl-cyclopentyl-1- Carboxylic acid L-isoleucine ester (compound 1-2)
[0038]
[0039] The synthetic method is the same as above, and the yield is 48.1%.
Embodiment 3
[0040] Example 3 In vivo pharmacokinetic studies
[0041] Experimental animals: Sprague-Dawley rats,
[0042] Administration method: Compounds A, 1-1 and 1-2 were intragastrically administered to rats at a dose of 15 mg / kg.
[0043] Rats were injected with Compound A through the tail vein at a dose of 4 mg / kg. The doses are all calculated according to compound A.
[0044] The concentration of Compound A in the plasma was determined after blood was taken from the rats. The results are shown in the table below:
[0045] The experimental results are shown in the table below. Average AUC after intravenous injection of Compound A (4 mg / kg) in rats 0-t It is 25.24 μg·h / mL. It can be seen from the table that after oral administration of compounds 1-1 and 1-2 to rats, the oral bioavailability of compound A was 61.2% and 43.1%, respectively.
[0046] Table 1 Pharmacokinetic parameters after oral administration of compound A, compound 1-1 and 1-2 in rats
[0047]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com