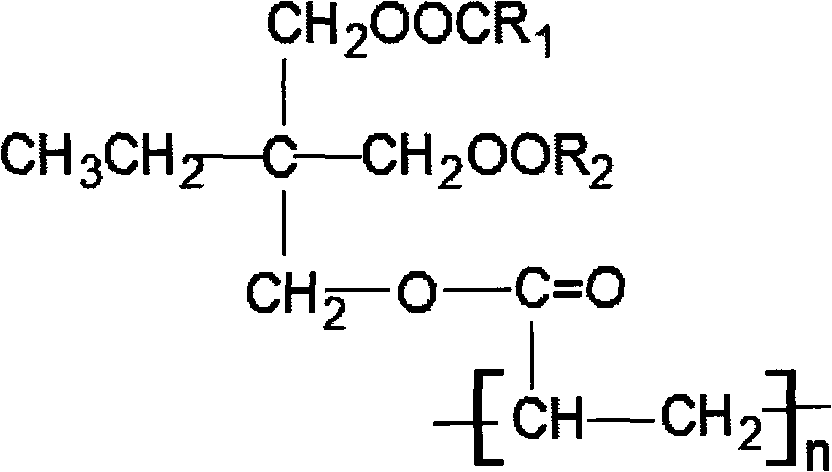
Trimethylolpropane-acrylic ester-based polyester and preparation method of polyester
A technology of trimethylolpropane and acrylate, applied in the direction of base material, petroleum industry, lubricating composition, etc., can solve the problems of increasing processing cost, reducing viscosity index, application limitation of ester products, etc., and achieving excellent lubricating properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take by weighing trimethylolpropane 42.3g (content 95%) and 21.6g acrylic acid, drop in the 250ml there-necked bottle of band electric stirring, thermometer, and vacuum distillation device, add catalyzer p-toluenesulfonic acid 1g again, control reaction temperature at 60°C, negative pressure reaction, control the vacuum degree to 0.01MPa, and the reaction time is 4h, to obtain trimethylolpropane-monoacrylate;
[0024] Then add 78.2g of heptanoic acid in molar ratio to the system, control the reaction temperature at 110°C, pump negative pressure to react, control the vacuum degree to 0.01MPa, the reaction time is 4h, cool to room temperature, and obtain the mixed ester of trimethylolpropane ;
[0025] Add 2 g of activated clay and 1 g of sodium carbonate to the system, stir evenly, and then filter to remove the catalyst p-toluenesulfonic acid to obtain a clean mixed ester of trimethylolpropane-2 heptanoate-1 acrylate with low acid value.
[0026] Add 0.5 g of initiator ...
Embodiment 2
[0029] Take by weighing trimethylolpropane 42.3g (content 95%) and 21.6g acrylic acid, add in the 250ml there-necked bottle with electric stirring, thermometer, and vacuum distillation device, add catalyst p-toluenesulfonic acid 1g again, control reaction temperature at 70°C, negative pressure reaction, control the vacuum degree to 0.02MPa, and the reaction time is 6h, to obtain trimethylolpropane-monoacrylate;
[0030] Then add 78.2g of heptanoic acid in molar ratio to the system, control the reaction temperature at 135°C, pump negative pressure to react, control the vacuum degree to 0.02MPa, the reaction time is 6h, cool to room temperature, and obtain the mixed ester of trimethylolpropane ;
[0031] Add 2 g of active clay and 1 g of sodium carbonate to the system, stir evenly and remove the catalyst p-toluenesulfonic acid by filtration to obtain a mixed ester of clean trimethylolpropane-2 heptanoate-1 acrylate with low acid value;
[0032] Add 0.5g of initiator azobisisobu...
Embodiment 3
[0034] Take by weighing trimethylolpropane 42.3g (content 95%) and 21.6g acrylic acid, add in the 250ml there-necked bottle with electric stirring, thermometer, and vacuum distillation device, add catalyst p-toluenesulfonic acid 1g again, control reaction temperature at 60°C, negative pressure reaction, control the vacuum degree to 0.01MPa, and the reaction time is 6h, to obtain trimethylolpropane-monoacrylate;
[0035] Then add 86.5g of molar caprylic acid to the system, control the reaction temperature at 140°C, pump negative pressure to react, control the vacuum degree to 0.01MPa, the reaction time is 6h, cool to room temperature, and obtain the mixed acid ester of trimethylolpropane ;
[0036] Add 2 g of activated clay and 1 g of sodium carbonate to the system, stir evenly and remove the catalyst p-toluenesulfonic acid by filtration to obtain a mixed ester of clean trimethylolpropane-2 caprylate-1 acrylate with low acid value;
[0037] Add 0.5 g of initiator azobisisobuty...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
| Open flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com