Iloperidone oral disintegrating tablet and its preparation method
A technology of iloperidone and orally disintegrating tablets is applied in the field of orally disintegrating tablets of antipsychotic pharmaceutical preparations and its preparation field, which can solve the problems of affecting oral taste, toxicity and the like, and achieves good taste, rapid disintegration and suitable for large The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
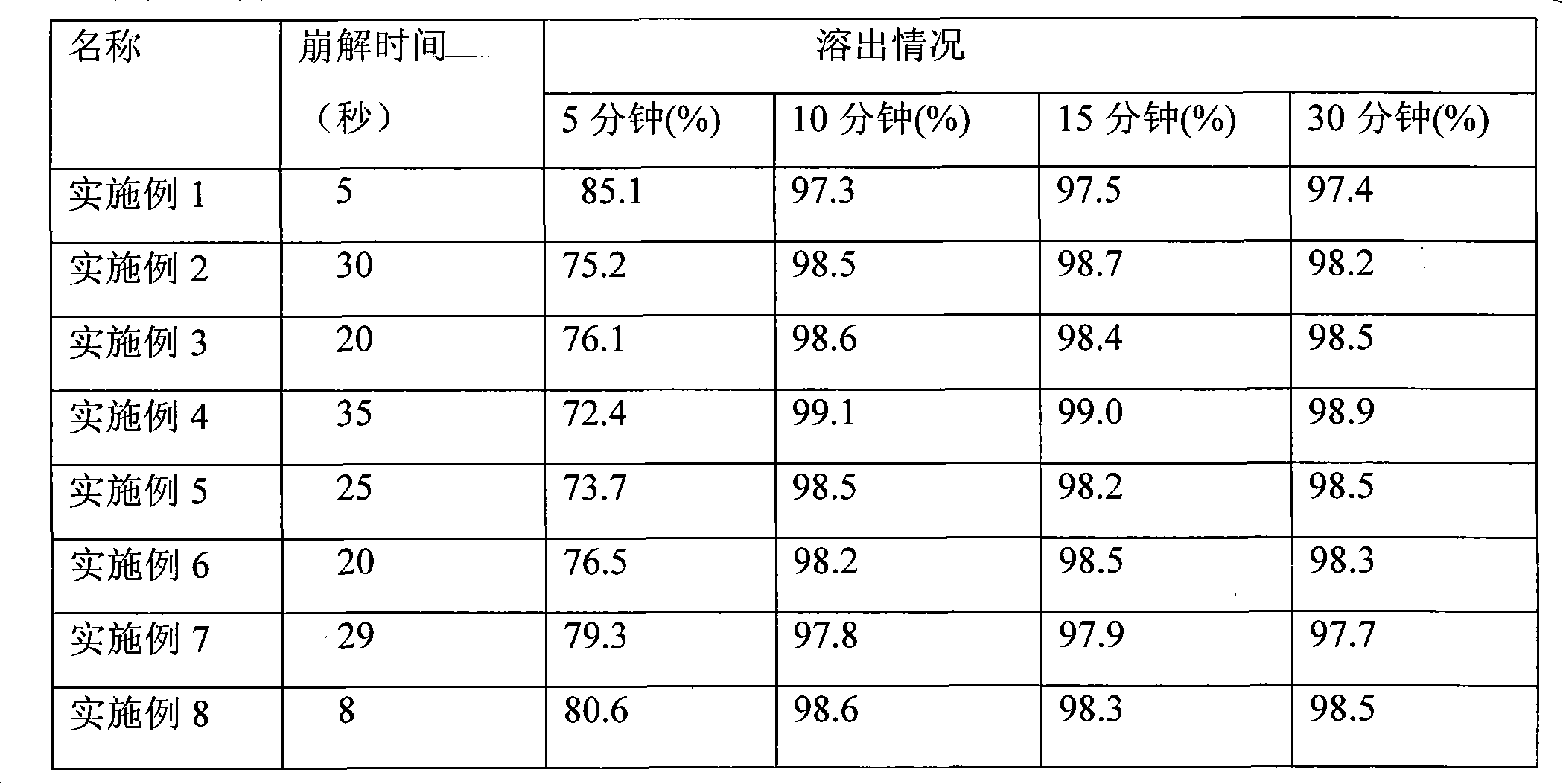
[0053] According to the prescription ratio of this embodiment (iloperidone: mannitol is 12: 20), take iloperidone 24g and mannitol 40g and mix evenly, and jet milling will control the particle size range at 99% less than 45 microns, and 90% less than 45 microns. 20 microns, spare. Take by weighing 32g of pulverized standby materials, prepare 20 mesh granules with 5% hypromellose aqueous solution as a binder, and dry for subsequent use; then weigh 20g of microcrystalline cellulose and 30g of xylitol and mix evenly, and mix them with 5% hypromellose % hypromellose aqueous solution is prepared into 20 mesh granules as a binder, and is dried for subsequent use; finally, 2 g of aspartame, 1 g of orange essence, 8 g of cross-linked pvp, 1 g of magnesium stearate, and micropowder are added to the two spare granules. 1g of silica gel, mixed evenly, controlled pressure 45N, tablet weight 95mg, the theoretical amount is 1000 tablets.
[0054] Appearance: Good
[0055] Taste: excellent...
Embodiment 2
[0057] According to the prescription ratio of this embodiment (iloperidone: xylitol is 12:30), take iloperidone 24g and mannitol 60g and mix evenly, jet milling will control the particle size range at 99% less than 45 microns, 90% Less than 20 microns, spare. Weigh 42g of crushed spare material, prepare 24 mesh granules with 5% hypromellose aqueous solution as a binder, and dry for standby; then weigh 20g of microcrystalline cellulose and 30g of mannitol and mix evenly with 5% hypromellose The hypromellose aqueous solution is used as a binder to prepare 24-mesh granules, which are dried for later use; finally, add 2g of AK sugar, 1g of orange essence, 8g of cross-linked pvp, 2g of talcum powder, and 1g of micronized silica gel to the two spare granules, and mix well , the control pressure is 37N, the tablet weight is 106mg, and the theoretical amount is 1000 tablets.
[0058] Appearance: good
[0059] Taste: Excellent
Embodiment 3
[0061] According to the prescription ratio of this embodiment (iloperidone: sorbitol is 12:40), take iloperidone 24g and mannitol 80g and mix evenly, jet milling will control the particle size range at 99% less than 45 microns, and 90% less than 45 microns. 20 microns, spare. Weigh 52g of the pulverized spare material, prepare 24 mesh particles with 5% hypromellose aqueous solution as a binder, and dry for standby; then weigh 20g of microcrystalline cellulose, 10g of lactose, and 30g of mannitol and mix evenly. Use 5% hypromellose aqueous solution as a binder to prepare 24-mesh granules, dry them for later use; finally add 2 g of AK sugar, 1 g of mint flavor, 15 g of sodium carboxymethyl starch, and magnesium stearate to the two spare granules 3g, 1g of micro-powdered silica gel, mixed evenly, controlled pressure 42N, tablet weight 134mg tablet, the theoretical amount is 1000 tablets.
[0062] Appearance: good
[0063] Taste: excellent
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com