Preparation method of epsilon-N-lauroyl lysine
A technology of lauroyl lysine and lauroyl lysine, which is applied in the field of preparation of ε-N-lauroyl lysine, can solve problems such as troublesome post-processing, inability to obtain high quality, etc. Simple process and clear reaction direction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
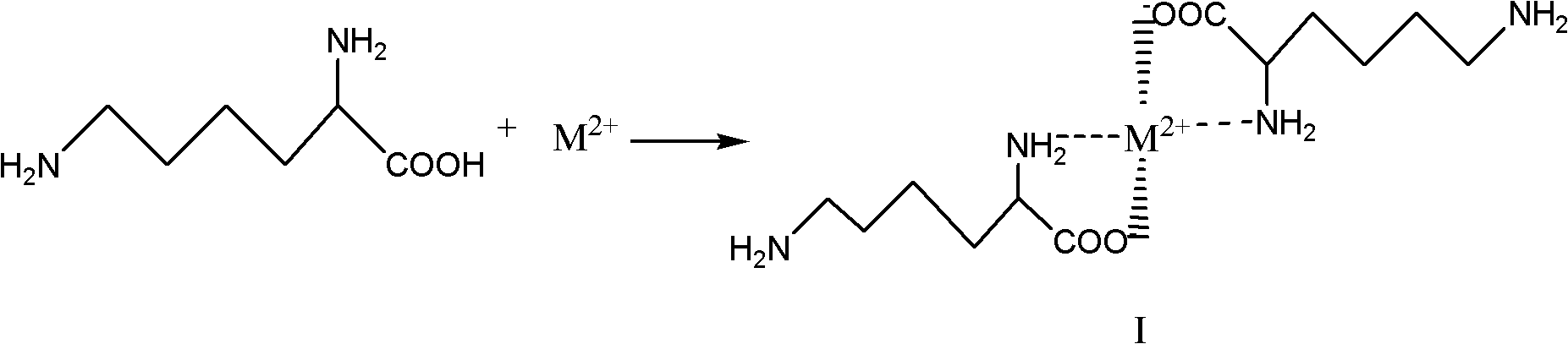
[0015] ① Chelate preparation:
[0016] The preparation of chelate can be expressed as follows by chemical reaction equation:
[0017]
[0018] In the above formula, M 2+ Represents a divalent metal ion, which can be Ca 2 +, Mg 2+ 、Cu 2+ , Fe 2+ , Zn 2+ Wait. The compound of formula I is a chelate.
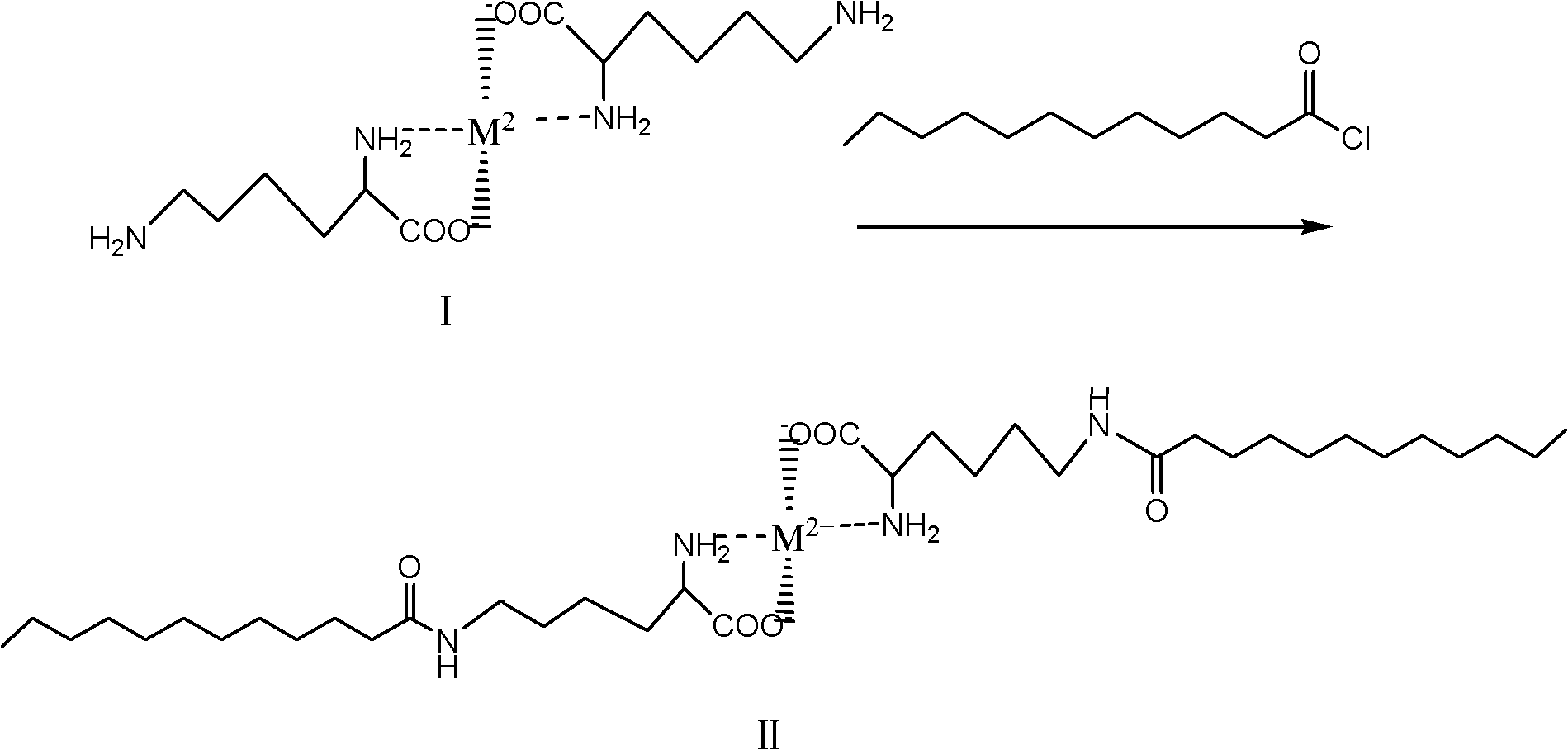
[0019] ② The chelate represented by formula I and lauroyl chloride carry out amidation reaction to obtain the compound of formula II.
[0020]
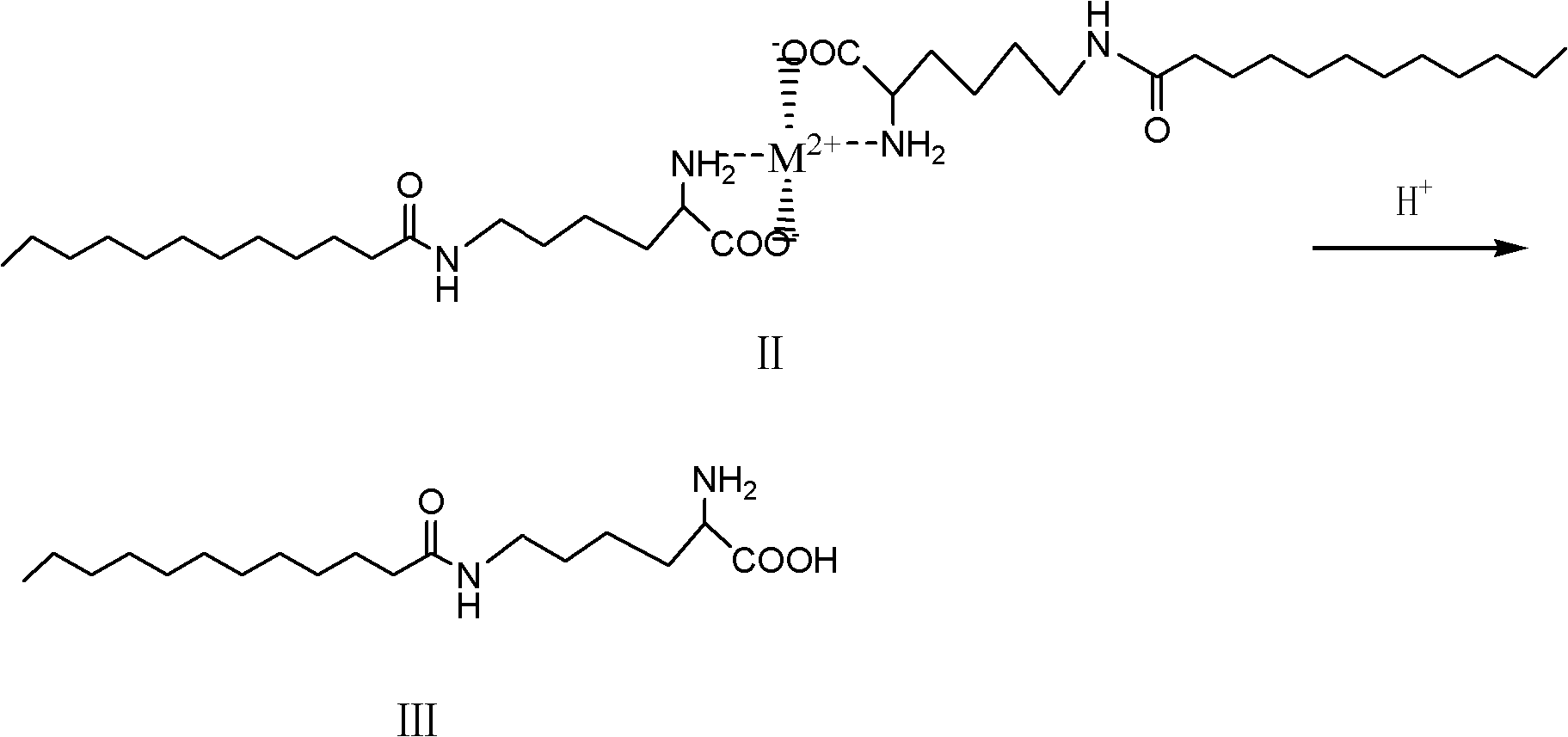
[0021] ③Acid hydrolysis of the compound of formula II to generate ε-N-lauroyl lysine (compound of formula III).
[0022]
Embodiment 1
[0025] In a reaction flask equipped with a stirrer, add CaCl 2 111g (1mol) and water, stir to dissolve, then add 292g (2mol) of L-lysine in batches, stir for 3 hours, adjust the pH to 8.0, dissolve the material in the bottle with salt water and cool to about -10°C, and start adding dropwise 218g (1mol) of lauroyl chloride was dropped within 2 hours. After the drop was completed, the reaction was continued at this temperature for 6 hours to obtain a white solid.
[0026] Add the white solid obtained above to 30wt% hydrochloric acid solution, stir at room temperature for 4 hours, filter to obtain the crude product, wash the crude product with purified water three times, and finally dry it to obtain a refined product with a melting point of 229-230°C, ε-N -Lauroyl lysine is 98.6% pure.
Embodiment 2
[0028] In a reaction flask equipped with a stirrer, add MgCl 2 95g (1mol) and water, stir to dissolve, then add 146g (1mol) of L-lysine in batches, stir for 3 hours, adjust the pH to 7.5, dissolve the material in the bottle with salt water and cool to about -5°C, and start adding dropwise 218g (1mol) of lauroyl chloride was dropped within 2 hours. After the drop was completed, the reaction was continued at this temperature for 8 hours to obtain a white solid.
[0029] Add the resulting white solid to 30 wt% hydrochloric acid solution, stir at room temperature for 5 hours, filter to obtain the crude product, wash the crude product with purified water three times, and finally dry it to obtain a refined product with a melting point of 229-230°C, ε-N- The purity of lauroyl lysine is 98.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com