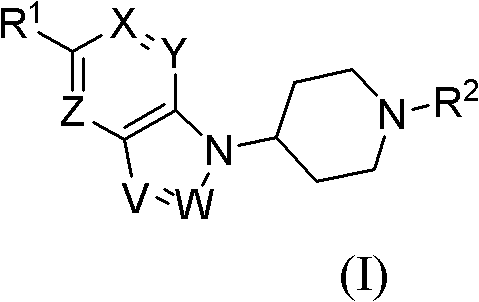
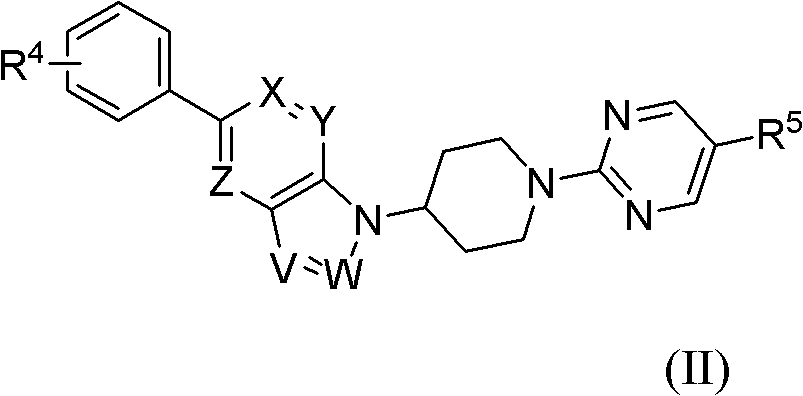
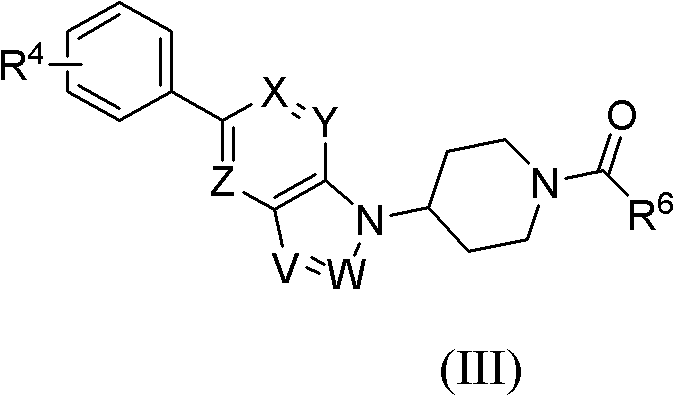
Bicycloheteroaryl compounds as GPR Receptor stimulant, compositions and application thereof
A compound, heteroaryl technology, applied in the field of bicyclic heteroaryl compounds, can solve problems such as hypoglycemia, failure to achieve accurate standardization of blood glucose levels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] 1-(1-(5-Ethylpyrimidin-2-yl)piperidinyl-4-yl)-5-(4-(methylsulfonyl)phenyl)-1hydro-indole
[0150]
[0151] Step 1: 4-Hydroxy-(2-(5-ethylpyrimidinyl))piperidine
[0152]
[0153] To 4-hydroxypiperidine (2.55g, 25.2mmol) in acetonitrile (50mL) was added 2-chloro-5-ethylpyrimidine (3.00g, 21.0mmol) and N,N-diisopropylethylamine ( 3.00g, 21.0mmol). The reaction solution was heated at 80°C for 16 hours, and then diluted with water. The resulting mixture was extracted with ethyl acetate, and the organic phase was washed with saturated brine, then dried and concentrated in vacuo. The residue was separated by silica gel column chromatography (dichloromethane: ethyl acetate = 3:1 to 1:1) to obtain the desired product (3.69 g, 85%).
[0154] 1 H NMR(CDCl 3 ): δ8.16 (2H, s), 4.37-4.42 (2H, m), 3.92-3.94 (1H, m), 3.24-3.30 (2H, m), 2.45 (2H, q, J=7.6 Hz), 1.92-1.98 (2H, m), 1.69 (1H, brs), 1.48-1.53 (2H, m), 1.19 (3H, t, J=7.6 Hz).
[0155] Step 2: (2-(5-Ethylpyrimidinyl)))piperidiny...
Embodiment 2
[0168] 6-(4-(1Hydroxy-tetrazol-1-yl)phenyl)-3-(1-(5-ethylpyrimidin-2-yl)piperidinyl)-3hydro-[1,2,3 ]Triazolo[4,5-b]pyridine
[0169]
[0170] Step 1: 1-(4-Bromophenyl)-1hydro-tetrazole
[0171]
[0172] Trimethyl orthoformate (1.14 g, 13.4 mmol) was added dropwise to a solution of 4-bromoaniline (2 g, 11.6 mmol) in acetic acid (12 mL) and stirred at room temperature for 1 hour. Then sodium azide was added and stirred at 80°C for 2 hours. After cooling, water and 6N (12 mL) hydrochloric acid (3.6 mL) were added, and a solution of sodium nitrite (0.64 g, 9.3 mmol) was added dropwise under an ice water bath. After stirring for 1 hour, it was filtered, washed with water, and dried to obtain the desired product (2.20 g, 98%).
[0173] 1 H NMR(CDCl 3 ): δ 8.98 (1H, s), 7.74 (2H, d, J=8.0 Hz), 7.61 (2H, d, J=8.0 Hz).
[0174] Step 2: 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1hydro-tetrazole
[0175]
[0176] To a solution of 1-(4-bromophenyl)-1hydro-tetrazole (1.0g, 4.44mm...
Embodiment 3
[0207] 5-(4-(1hydro-tetrazol-1-yl)phenyl)-1-(1-(5-ethylpyrimidin-2-yl)piperidin-4-yl)-1hydro-indole
[0208]
[0209] In a method similar to that described in Example 2 step 7 from 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene Yl)-1hydro-tetrazole (Example 2 step 2) and 5-bromo-1-(1-(5-ethylpyrimidin-2-yl)piperidin-4-yl)-1hydro-indole (Example 1, Step 3) The title compound was synthesized.
[0210] 1 H NMR(CDCl 3 )δ9.02 (1H, s), 8.23 (2H, s), 7.76-7.91 (5H, m), 7.49-7.56 (2H, m), 6.61-6.62 (1H, m), 5.00-5.04 (2H, m), 4.53-4.60 (1H, m), 3.05-3.14 (2H, m), 2.51 (2H, q, J=7.6 Hz), 2.21-2.25 (2H, m), 1.98-2.08 (2H, m) , 1.23 (3H, t, J=7.6 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com