Method for detecting 6-methylmercaptopurine
A detection method, the technology of methylmercapto, applied in the field of 6-purine detection, can solve the problems of complex 6-MMP method, and achieve the effects of high sensitivity, accurate results and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Preparation of anti-6-MMP specific antibody
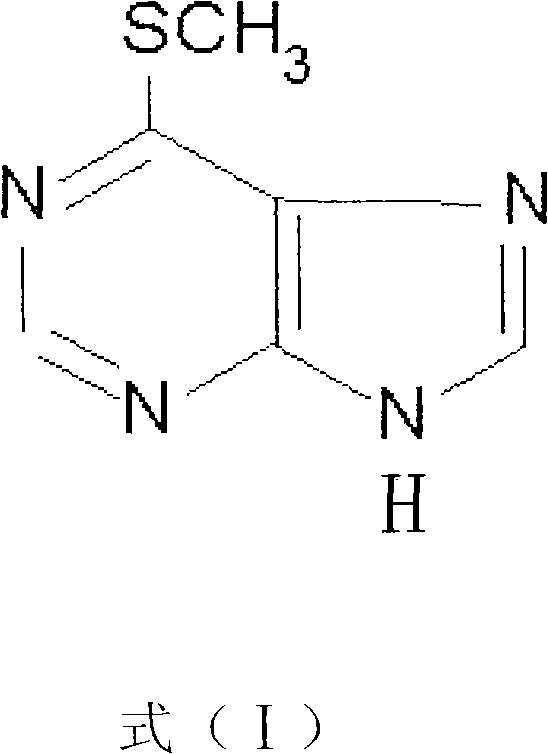
[0069] 1. Synthesis of 6-MMP derivatives, the chemical structure of which is shown in formula (II).
[0070]
[0071] The synthetic approach and method of this 6-MMP derivative are as follows:
[0072]
[0073] (1) Dissolve 5.0g 6-MMP and 4.45g K with 30ml DMF 2 CO 3 , add 10ml of DMF solution containing 4.06g of ethyl 6-bromohexanoate, and stir the reaction at 40°C for 30min.
[0074] (2) The reacted solution was then neutralized with 1N HCl solution, and extracted with ethyl acetate. The organic phase was dried, filtered, dried in vacuo and purified on normal phase silica gel. 1.5 g of the purified product was dissolved in 25 ml of methanol, then an aqueous solution containing 600 mg of LiOH.H2O was added, and the reaction was stirred at room temperature for 4 hours. After concentration, the product was diluted with water and washed with ether, and the pH of the aqueous phase solution was adjusted to 5...
Embodiment 2
[0083] Example 2 Preparation of homogeneous enzyme immunoassay reagent for 6-MMP
[0084] (1) Preparation of R1 reagent: Dilute the prepared antibody into R1 buffer, the homogeneous R1 buffer contains 50 mM Tris, 0.25% BSA, 50 mM G-6-P and 50 mM NAD. The volume ratio of antibody to R1 buffer was 1:1000.
[0085] (2) Preparation of R2 reagent
[0086] 1) Preparation of G6PDH-6MMP
[0087] a) Weigh 15mg G6PDH, dissolve it in 12ml, 0.05M Tris buffer, add 100mg NADH, 0.5ml carbitol and 1ml DMF in sequence and mix well;
[0088] b) Dissolve 10 mg of 6-MMP derivative in 420 μl dimethyl sulfoxide and 180 μl DMF, add 6 μl tributylamine and 3 μl isobutylchloroformate, and stir the reaction at 2-8°C 30min;
[0089]c) Stir overnight at 2-8° C., and purify the obtained G6PDH-6MMP.
[0090] 2) Dilute the prepared G6PDH-6MMP into R2 buffer. R2 buffer was 100 mM Tris, 0.25% BSA. The volume ratio of antibody to R2 buffer was 1:1000.
Embodiment 3
[0091] Example 3 Homogeneous enzyme immunoassay method and calibration results of 6-MMP
[0092] Table 1 Hitachi 7180 analyzer 6-MMP homogeneous enzyme immunoassay parameter list
[0093]
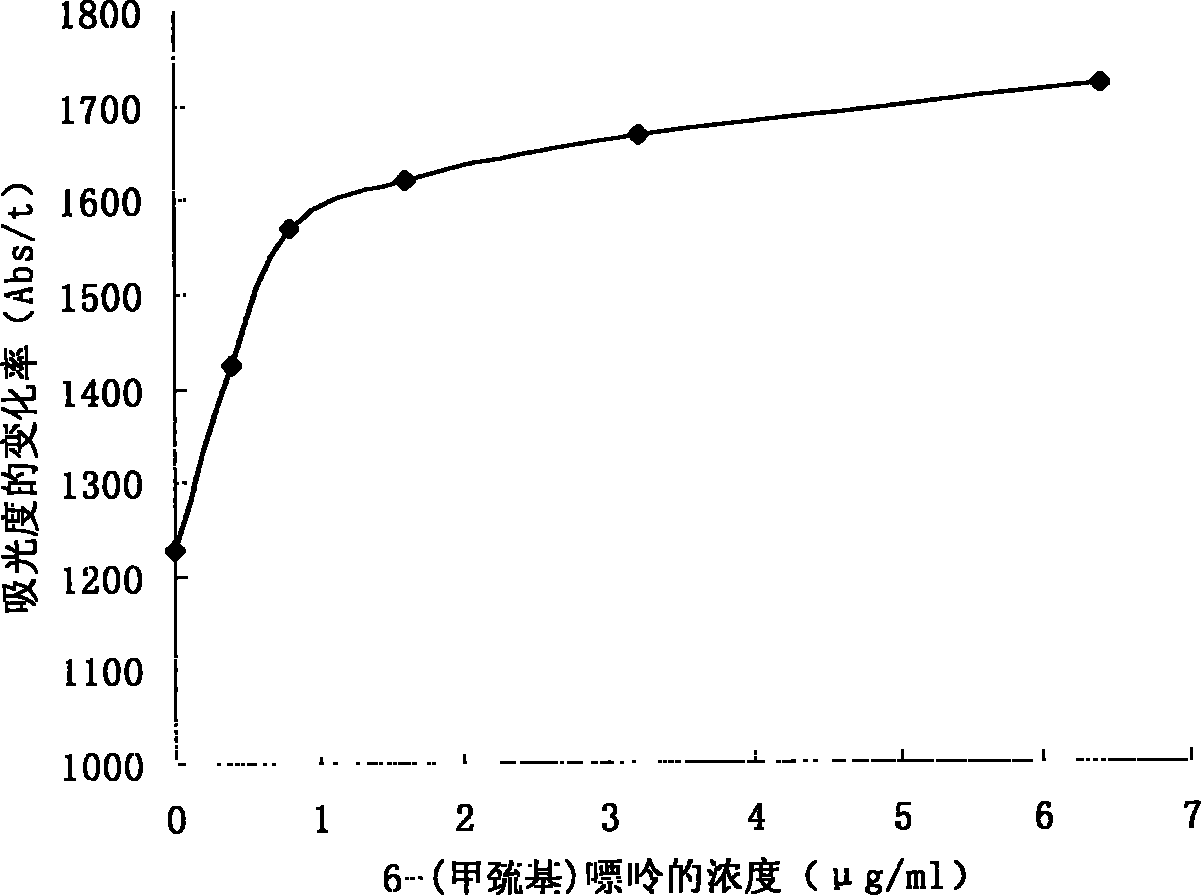
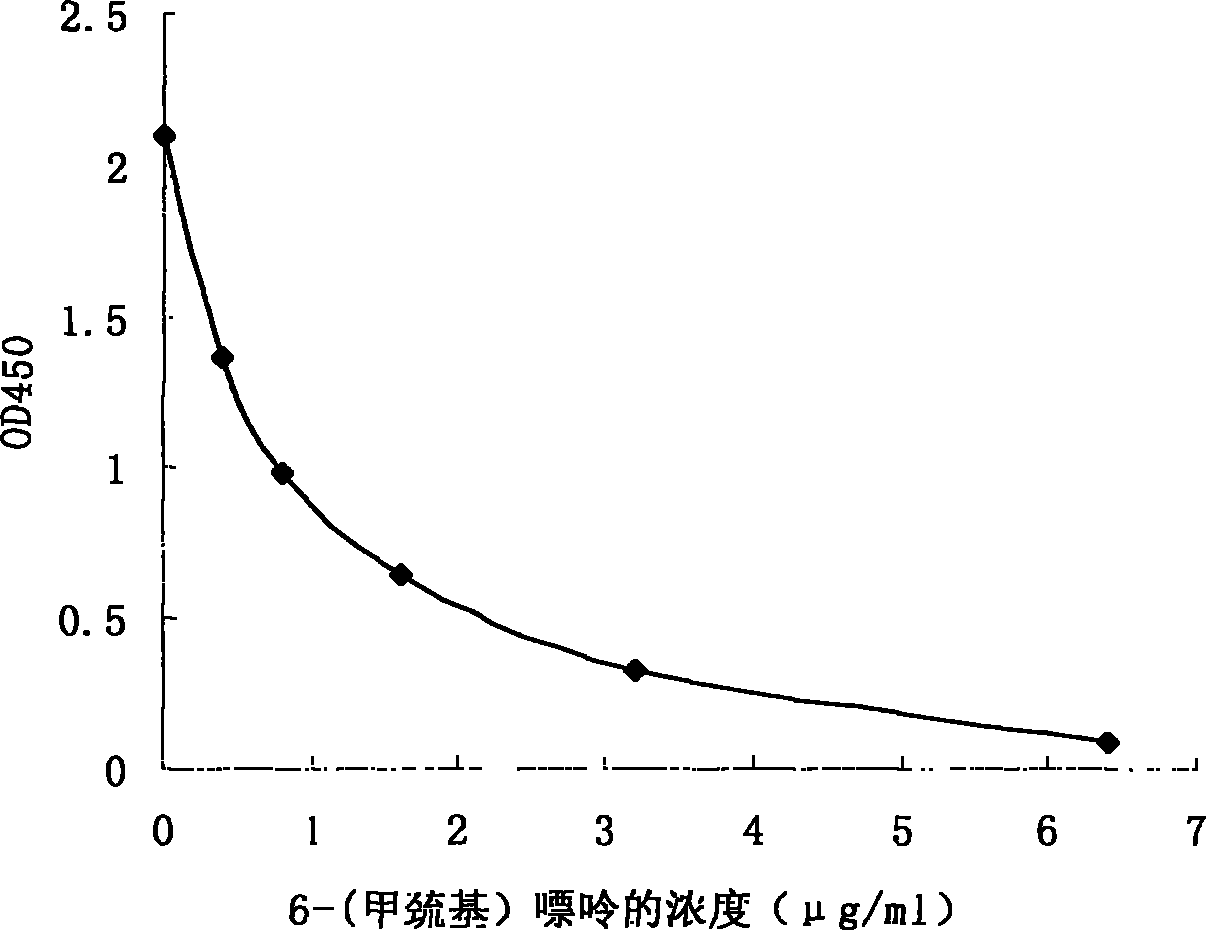
[0094] Through the automatic biochemical analyzer, set the parameters according to the data in Table 1. First add the sample, then add the R1 reagent (the mixture of the antibody and the R1 buffer) in Example 2, and finally add the R2 reagent (the mixture of the G6PDH-6MMP conjugate and the R2 buffer), and measure the OD340 at different time points Absorbance value, calculate the absorbance change rate of different concentrations of standard substances, and obtain a more ideal reaction standard curve, the results are as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com