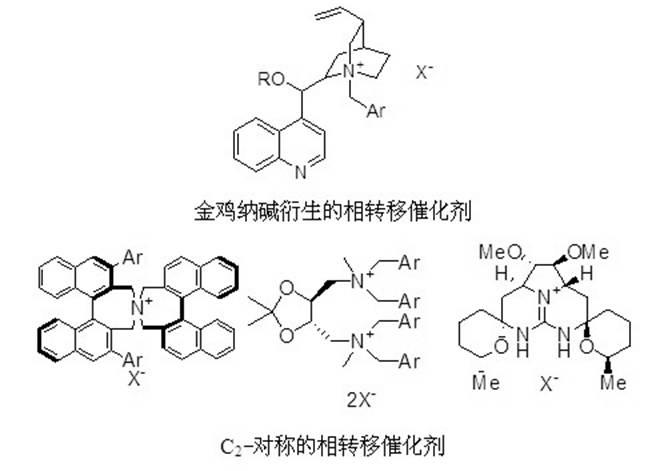
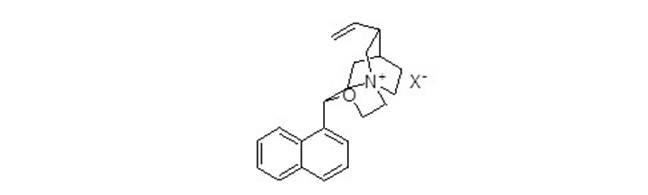
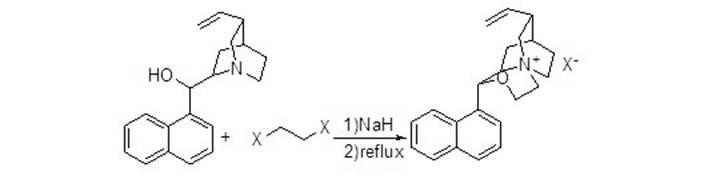
Chiral phase transfer catalyst and synthesizing method thereof
A synthesis method and catalyst technology, applied in the field of synthesis of new chiral phase transfer catalysts, can solve problems such as insufficient scope of application, difficult application, complex synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] In a reaction flask, add 0.391g (1.3mmol) of cinchonaline, dissolve it with 10ml of dimethylformamide (DMF), then add 0.518g (13mmol) of NaH, react for 2 hours, and then drop in dibromoethane 0.26 g (1.43mmol), reacted at room temperature for 2 hours, and raised the temperature to 60°C for 2 hours. After the reaction, added water, extracted with ethyl acetate, separated the organic layer, dried, and spun off the solvent to obtain the catalyst.
example 2
[0021] In a reaction flask, add 0.391g (1.3mmol) of cinchonaline, dissolve it with 10ml of tetrahydrofuran (THF), then add 0.518g (13mmol) of NaH, react for 2 hours, then drop into 0.26g (1.43mmol) of dibromoethane ), react at room temperature for 2 hours, and raise the temperature to 60°C for 2 hours. After the reaction, add water, extract with ethyl acetate, separate the organic layer, dry, and spin off the solvent to obtain the catalyst.
[0022] 2. Application: for asymmetric alkylation of glycine tert-butyl ester derivatives
[0023] example 1:
[0024] In the reaction bottle, add 5.68 mg of the prepared catalyst, dissolve it in 2 ml of toluene, add 0.1 g of 50% potassium hydroxide, stir for 1 hour, and add 29.6 mg of glycine tert-butyl ester derivative ( 2-Dibenzyl iminoacetate tert-butyl), and react at this temperature for 1 hour, then add 12 μL of alkylating reagent, and keep the temperature at 0 ℃ for reaction, after the reaction is completed, add water, and dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com