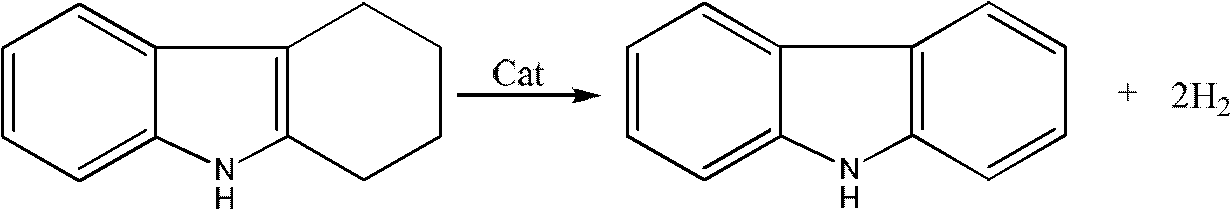
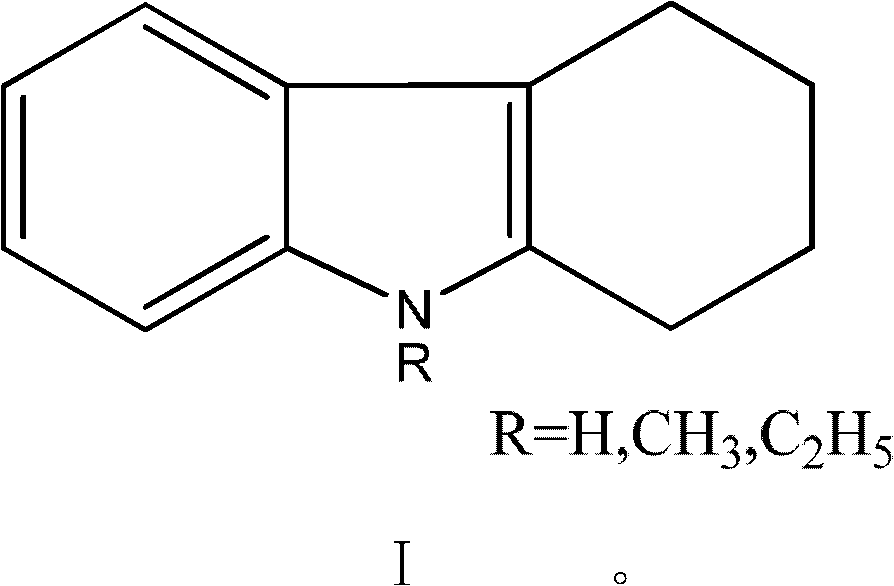
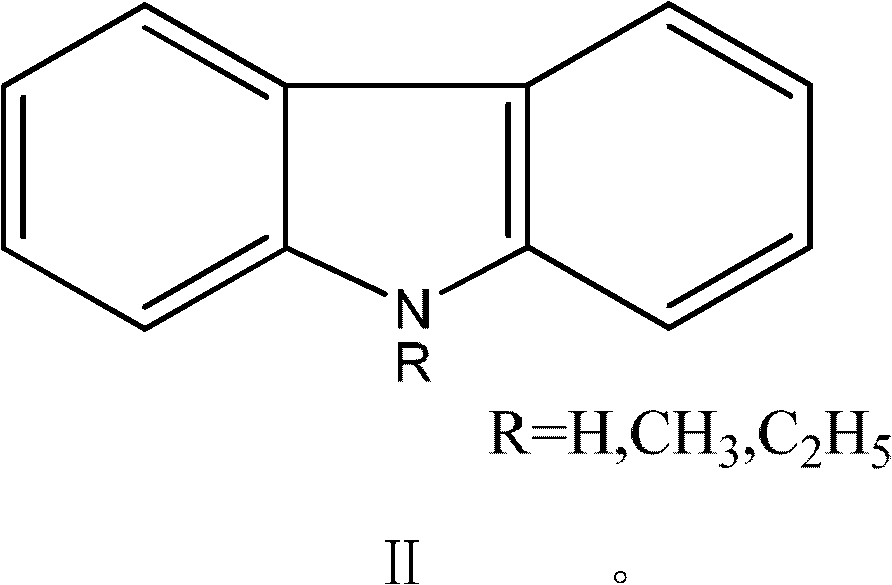
Method for synthesizing carbazole compounds
A technology of compound and carbazole, which is applied in the field of synthesizing carbazole compounds, can solve the problem of high cost and achieve the effects of low cost, high reaction yield and product purity, high conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In a 1000ml three-necked flask equipped with a reflux condenser, add 1,2,3,4-tetrahydrocarbazole 112g (0.66mol), 400g solvent N,N-dimethylformamide successively, add 3.0g activated carbon, Warm to reflux. Heat preservation reaction for 3.0h, filter, wash the catalyst with 50ml hot solvent, combine the filtrate, add 0.5g 10% platinum / mesoporous carbon to it after the filtrate is cooled, raise the temperature to reflux, heat preservation reaction for 5h, filter out the catalyst, distill and concentrate the filtrate, add water , filtered, and dried to obtain 106 g of carbazole with a yield of 97% and a HPLC chromatographic purity of 99%.
Embodiment 2
[0051] In a 1000ml three-necked flask equipped with a reflux condenser, 112g (0.66mol) of 1,2,3,4-tetrahydrocarbazole, 400g of solvent N,N-dimethylacetamide were added successively, and 3.0g of diatomaceous earth was added , warmed to reflux. Heat preservation reaction for 3.0h, filter, 50ml hot solvent washes the catalyst, combine the filtrate, after the filtrate is cooled, add 0.5g 10% ruthenium / activated carbon to it, heat up to reflux, heat preservation reaction for 7h, filter out the catalyst, distill and concentrate the filtrate, add water, After filtering and drying, 105 g of carbazole was obtained with a yield of 96% and a HPLC chromatographic purity of 98.5%.
Embodiment 3
[0053] In a 1000ml three-necked flask equipped with a reflux condenser, 112g (0.66mol) of 1,2,3,4-tetrahydrocarbazole, 400g of solvent polychlorobenzene, and 3.0g of Raney nickel were added in sequence, and the temperature was raised to reflux. Heat preservation reaction for 3.0 hours, filter, wash the catalyst with 50ml of hot solvent, combine the filtrate, add 0.5g 10% ruthenium / alumina to it after the filtrate is cooled, heat up to reflux, heat preservation reaction for 8 hours, distill and concentrate the filtrate, add water, filter and dry , to obtain 103g carbazole, yield 94%, HPLC chromatographic purity 98.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com