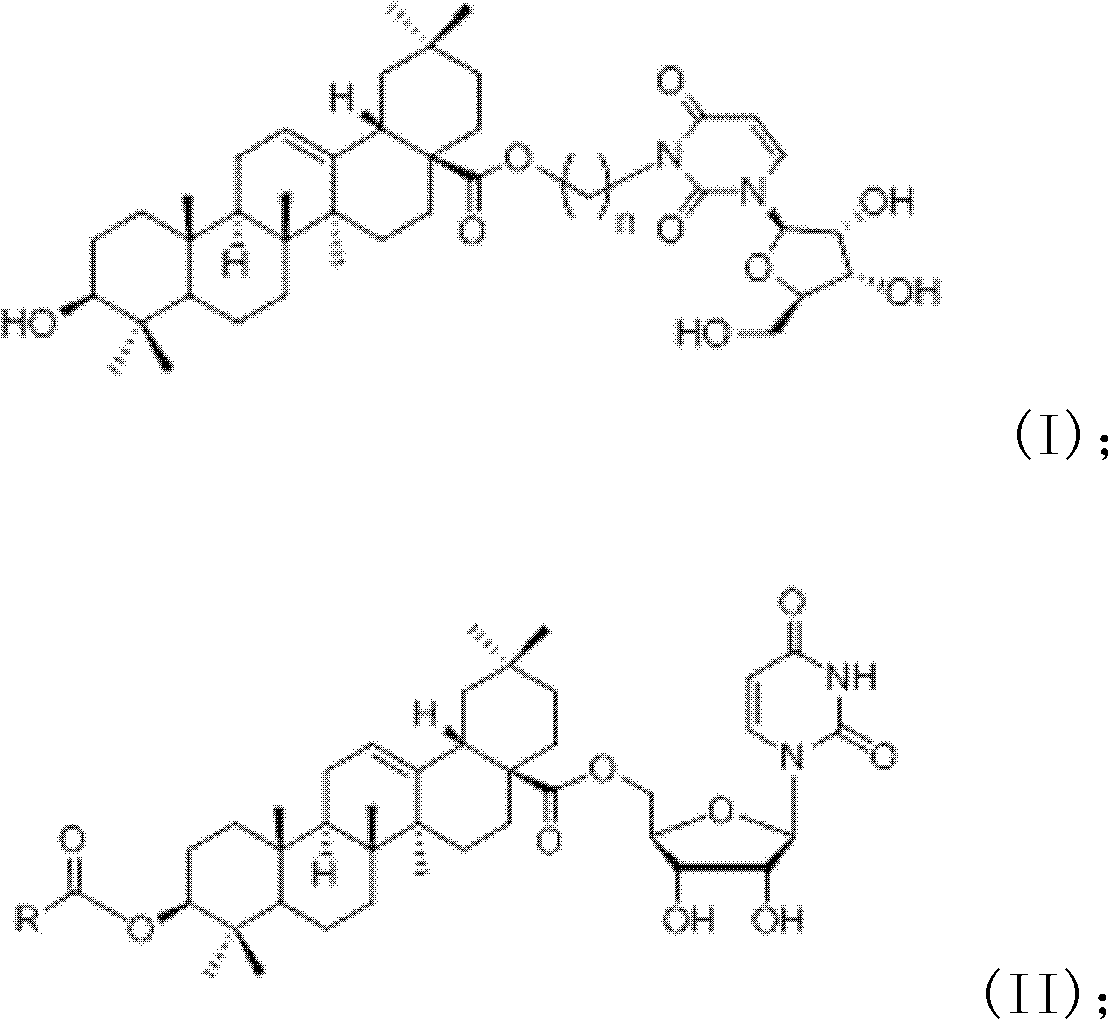
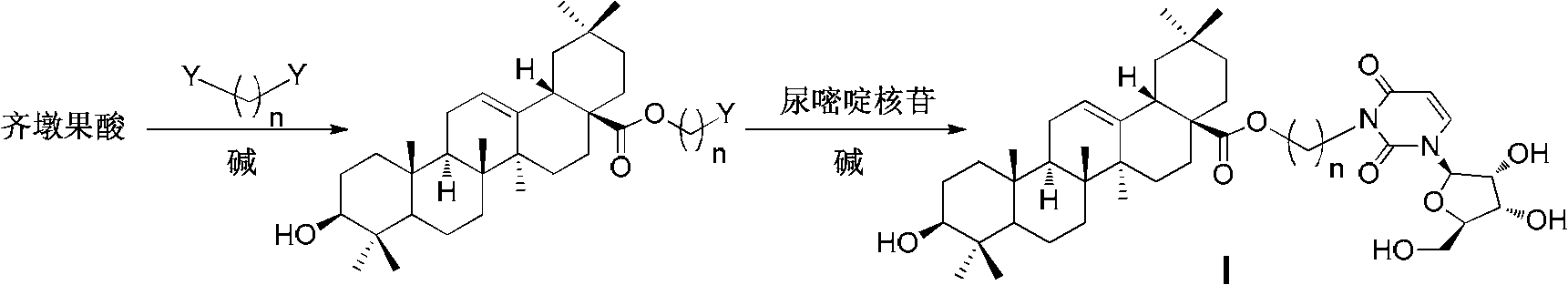
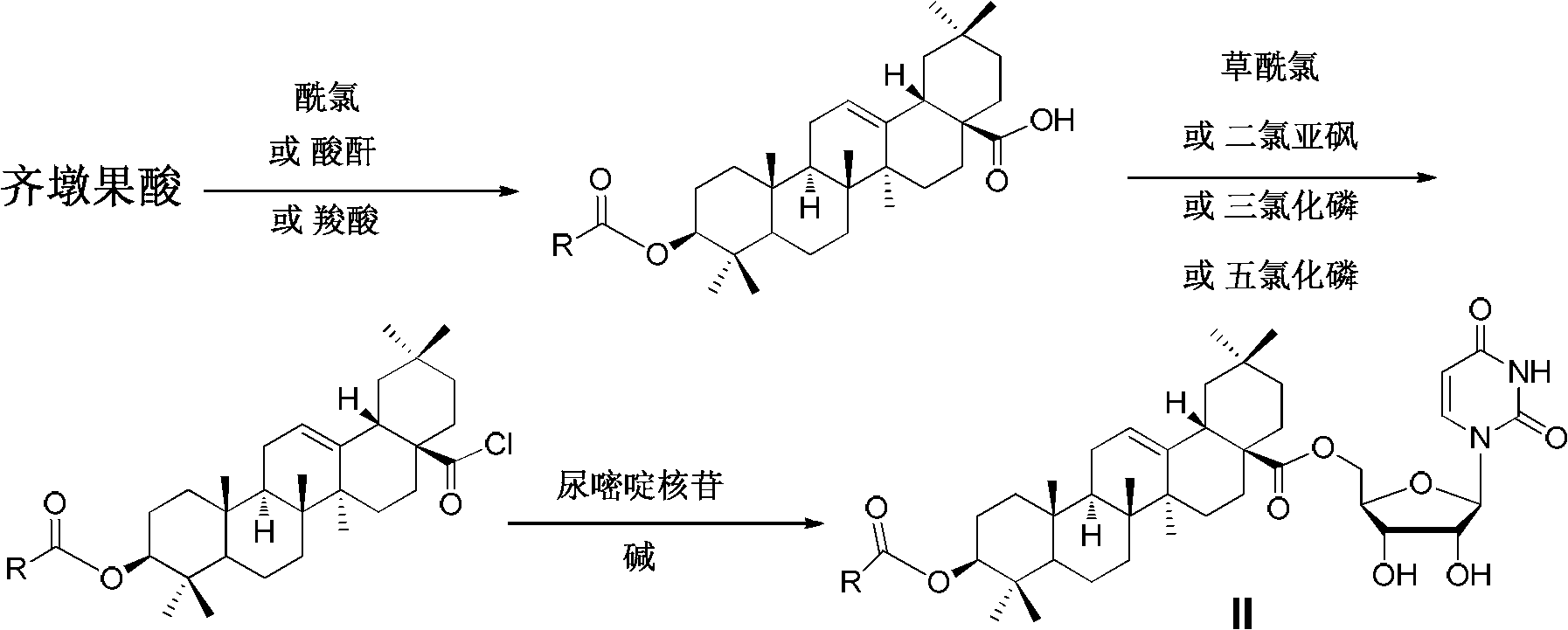
Oleanolic acid-uridine conjugate as well as preparation method and application thereof
A technology of uridine nucleoside conjugates and uridine nucleoside esters, applied in the field of medicine, can solve the problems of weak anti-tumor activity, obstacles and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation of 3β-hydroxy-oleanane-12-ene-28-acid-[(uridine-3)-ethyl]ester (I1)
[0071] a) Dissolve 4.99 mmol (2.28 g) of oleanolic acid in 10 mL of DMF, add 24.95 mmol (3.44 g) of potassium carbonate and 5.99 mmol (0.52 mL) of 1,2-dibromoethane, and stir at room temperature for 12 hours, The solvent was spun off under reduced pressure, and the residue was dissolved in 50 mL of ethyl acetate, washed successively with 1N HCl, water, saturated sodium bicarbonate, water and saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated. Purified by silica gel column chromatography, eluted with a mixed solvent of petroleum ether: ethyl acetate = 6: 1 (volume ratio), tracked and detected by thin layer chromatography, collected the eluate, and evaporated the eluate to dryness to obtain 3β-hydroxyl -Oleanane-12-ene-28-acid-(2-bromoethyl)ester 1.60g (white solid, yield 57%); 1 H NMR (500MHz, CDCl 3 )0.74, 0.78, 0.93, 0.99 and ...
Embodiment 2
[0077] Example 2: 3β-Hydroxy-oleanane-12-ene-28-acid-[(uridine-3)-n-butyl]ester (I 2 ) preparation
[0078] a) Dissolve 4.99mmol (2.28g) of oleanolic acid in 10mL of DMF, add 19.96mmol (2.76g) of potassium carbonate and 0.78mL (6.49mmol) of 1,4-dibromobutane, and stir at room temperature for 12 hours, The solvent was spun off, and the residue was dissolved in 50 mL of ethyl acetate, washed successively with 1N HCl, water, saturated sodium bicarbonate, water and saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated, and the resulting residue was passed through a silica gel column Purified by chromatography, eluted with a mixed solvent of petroleum ether: ethyl acetate = 6: 1 (volume ratio), tracked and detected by thin-layer chromatography, collected the eluate, evaporated the eluate to dryness, and obtained 3β-hydroxy-alcohol Aranane-12-ene-28-acid-(4-bromobutyl)ester 1.78g (white solid, yield 61%); 1 H NMR (500MHz, CDCl 3)0.72...
Embodiment 3
[0084] Example 3: 3β-Hydroxy-oleanane-12-ene-28-acid-[(uridine-3)-n-hexyl]ester (I 3 ) preparation
[0085] a) Dissolve 4.99 mmol (2.28 g) of oleanolic acid in 10 mL of DMF, add 9.98 mmol (1.38 g) of potassium carbonate and 6.24 mmol (0.96 mL) of 1,6-dibromohexane, and stir at room temperature for 12 hours, The solvent was spun off, and the residue was dissolved in 50 mL of ethyl acetate, washed successively with 1N HCl, water, saturated sodium bicarbonate, water and saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated, and the resulting residue was passed through a silica gel column Purified by chromatography, eluted with a mixed solvent of petroleum ether: ethyl acetate = 8: 1 (volume ratio), tracked and detected by thin layer chromatography, collected the eluate, evaporated the eluate to dryness, and obtained 3β-hydroxy-alcohol Aranane-12-ene-28-acid-(6-bromohexyl)ester 1.80g (white solid, yield 58%); 1 H NMR (500MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com