Process for the production of a sulfone monomer
A kind of technology of diphenyl sulfone and dichlorodiphenyl sulfone, which is used in the field of preparing sulfone monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
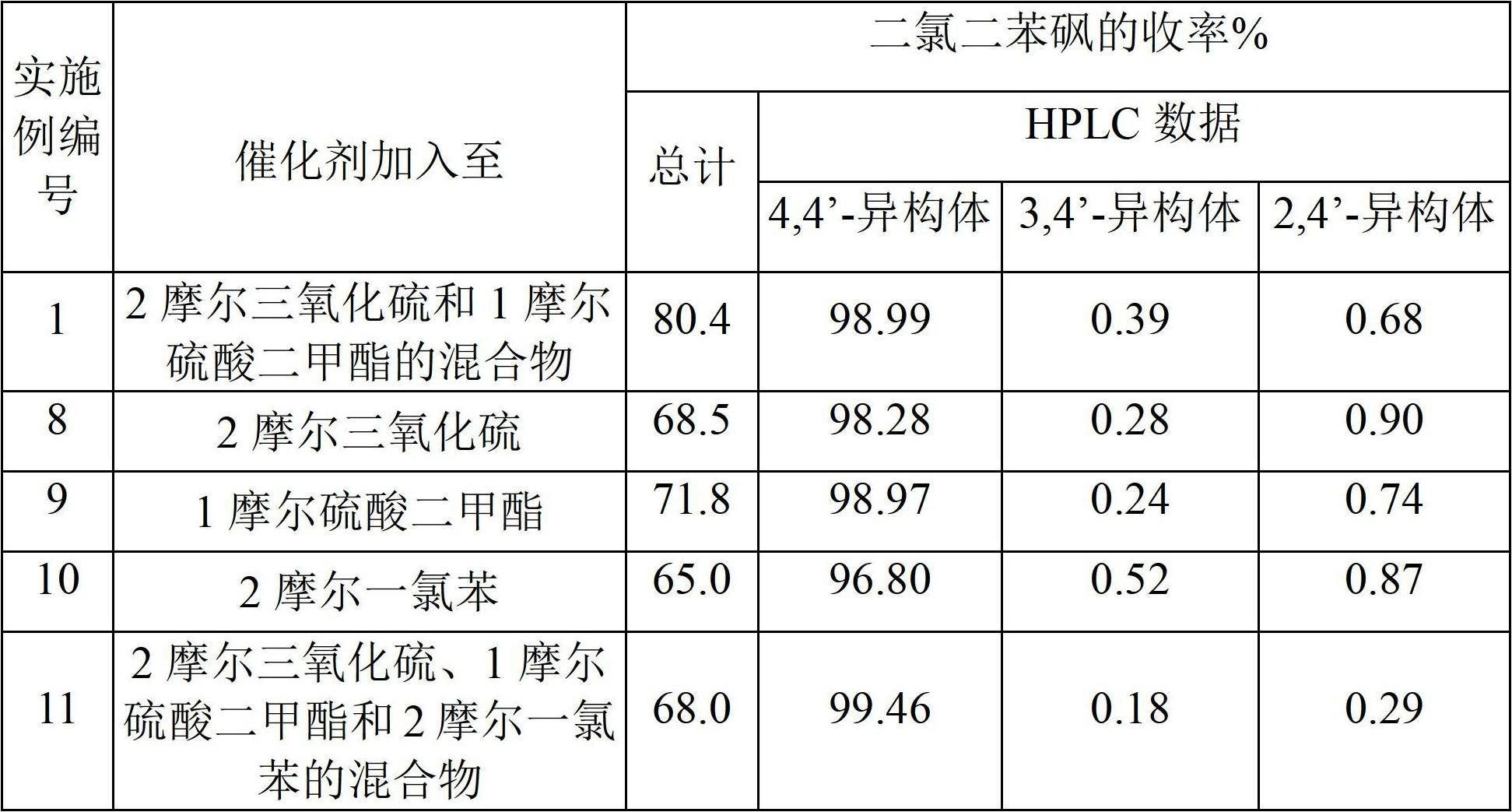
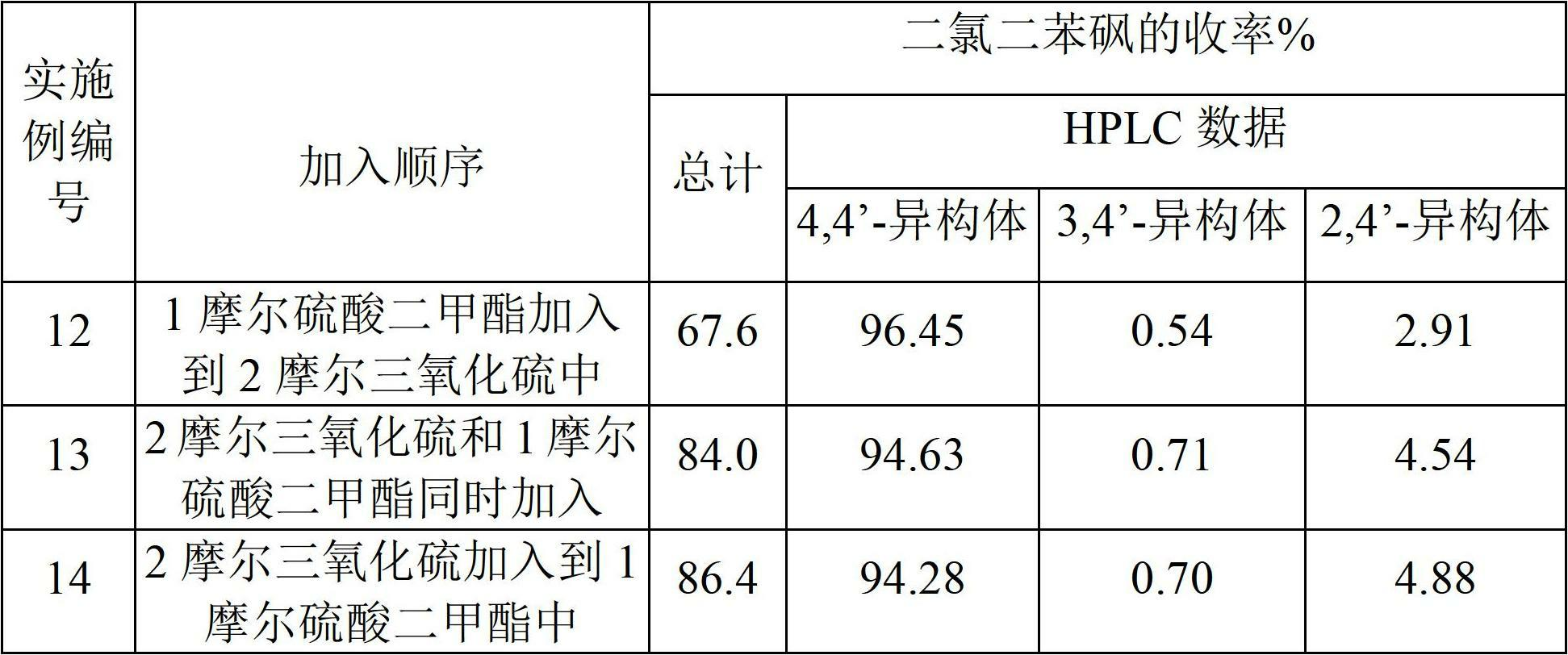
[0188] To prepare crude 4,4'-dichlorodiphenylsulfone, add sulfur trioxide to dimethyl sulfate in step 1.
[0189] 400 g of sulfur trioxide was added to 315 g of dimethyl sulfate over about 1 hour while maintaining the reaction mixture at 28°C-30°C for 30 minutes, followed by the addition of 1.0 g of boric acid and allowed to react for 30 minutes. The reaction mixture was added to 562 g of monochlorobenzene at 30°C-35°C over about 1 hour and held for 1 hour.
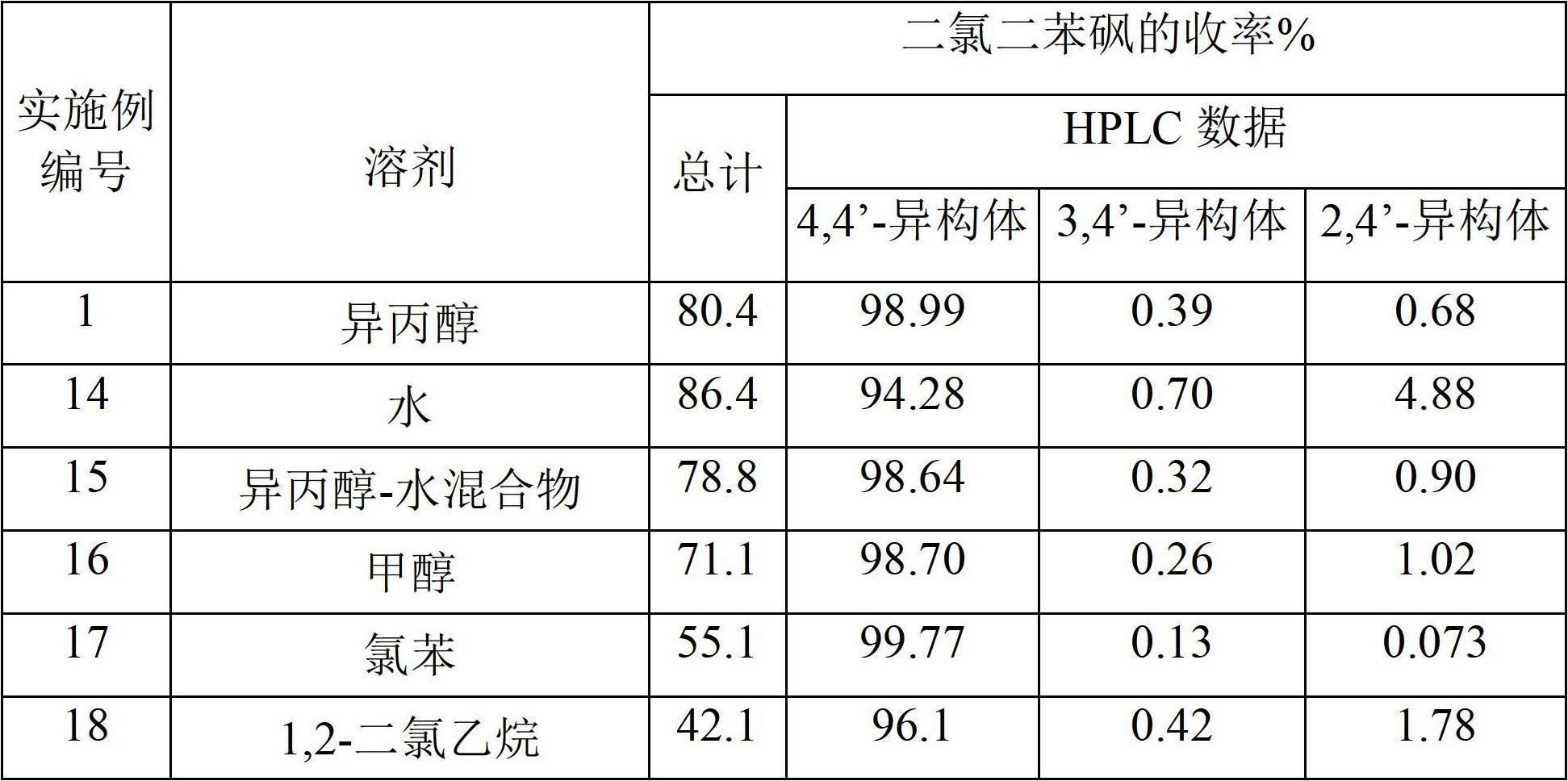
[0190] The reaction mixture was then added to 600 g of isopropanol to precipitate 4,4'-dichlorodiphenylsulfone. The precipitate was isolated and neutralized with caustic soda and washed repeatedly with water and dried to obtain 577 g of product (APHA 217). The yield was 80.4% relative to monochlorobenzene. HPLC analysis showed that the product composition was 98.99% of 4,4'-dichlorodiphenylsulfone, 0.39% of 3,4'-isomer and 0.68% of 2,4'-isomer. The yield of 4,4'-dichlorodiphenyl sulfone was 79.5% relative to monochloro...
Embodiment 2
[0192] Crude 4,4'-dichlorodiphenylsulfone was purified using a chelating agent.
[0193] 577 g of crude 4,4'-dichlorodiphenylsulfone was dissolved in a 1:1 mixture of 1160 g of chlorobenzene and water at 85°C-95°C. The resulting solution was treated with 1.2 g of chelating agent and the aqueous layer was separated. This step was repeated and the solvent layer was filtered off at 85°C-95°C. The filtrate was cooled to 30 °C to precipitate pure 4,4'-dichlorodiphenylsulfone, separated and washed with water and dried to obtain 542 g of product (APHA 8). The yield was 75.6% relative to monochlorobenzene. HPLC analysis showed that the product composition was 99.86% of 4,4'-dichlorodiphenylsulfone, 0.063% of 3,4'-isomer and 0.058% of 2,4'-isomer. The yield of 4,4'-dichlorodiphenyl sulfone was 75.5% relative to monochlorobenzene.
Embodiment 3
[0195] The crude 4,4'-dichlorodiphenylsulfone was purified by activated charcoal.
[0196] 400 g of sulfur trioxide was added to 315 g of dimethyl sulfate over about 1 hour while maintaining the reaction mixture at 28°C-30°C for 30 minutes, followed by the addition of 1.0 g of boric acid and allowed to react for 30 minutes. The reaction mixture was added to 562 g of monochlorobenzene at 30°C-35°C over about 1 hour and held for 1 hour.
[0197] The reaction mixture was then added to 600 g of isopropanol to precipitate 4,4'-dichlorodiphenylsulfone. The precipitate was isolated and neutralized with caustic soda and washed repeatedly with water and dried to obtain 570 g of product (APHA 237). The yield was 79.5% relative to monochlorobenzene. HPLC analysis showed that the product composition was 98.53% 4,4'-dichlorodiphenylsulfone, 0.40% 3,4'-isomer and 1.04% 2,4'-isomer. The yield of 4,4'-dichlorodiphenyl sulfone was 78.3% relative to monochlorobenzene.
[0198]570 g of crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com