Prodrugs of a piperidinyl derivative as modulators of chemokine receptor activity
A chemokine receptor and chemokine technology, applied in the fields of rheumatoid arthritis and transplant rejection, treatment and prevention of inflammatory diseases, allergic diseases and autoimmune diseases, can solve the problem of mobilizing monocytes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
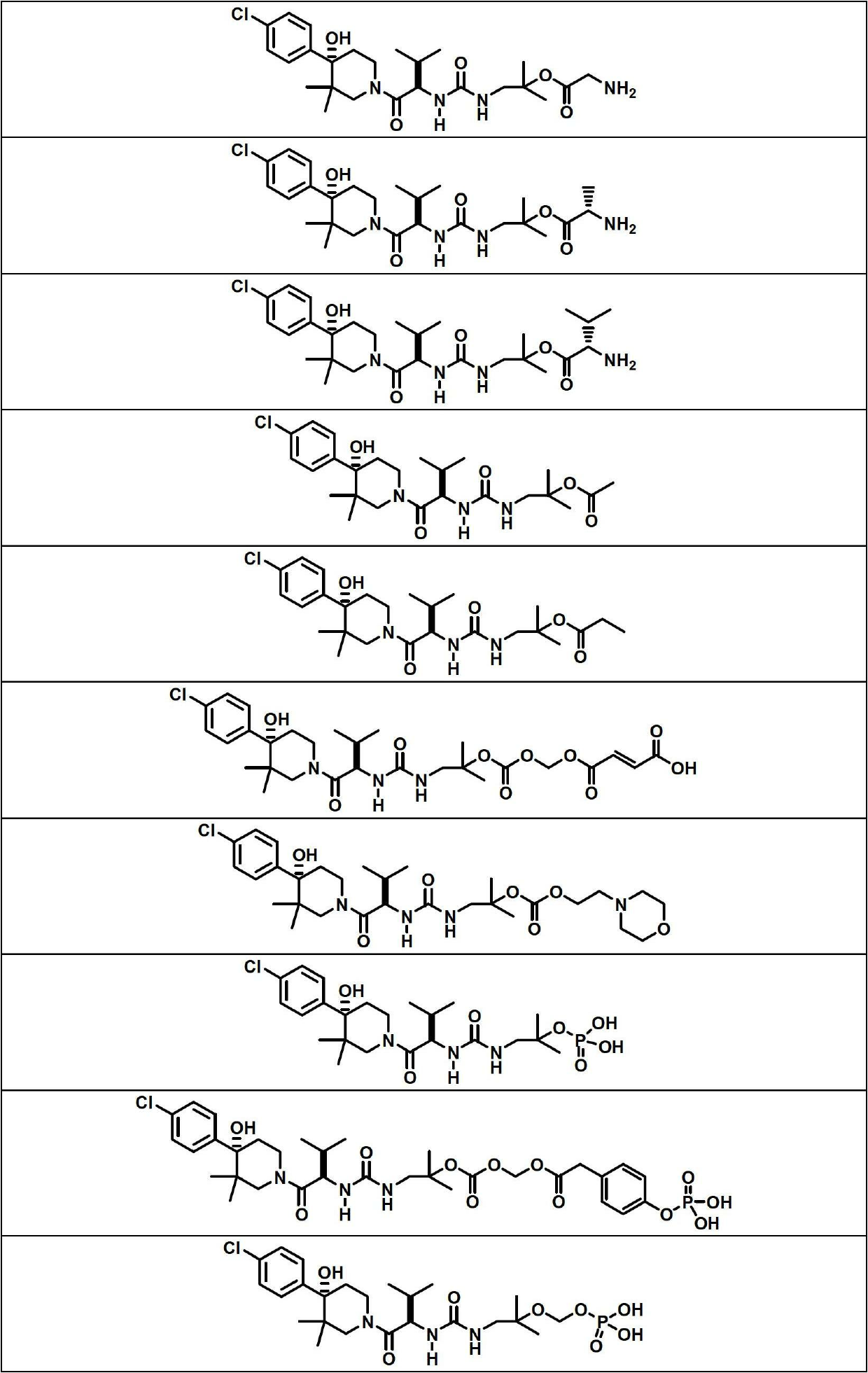
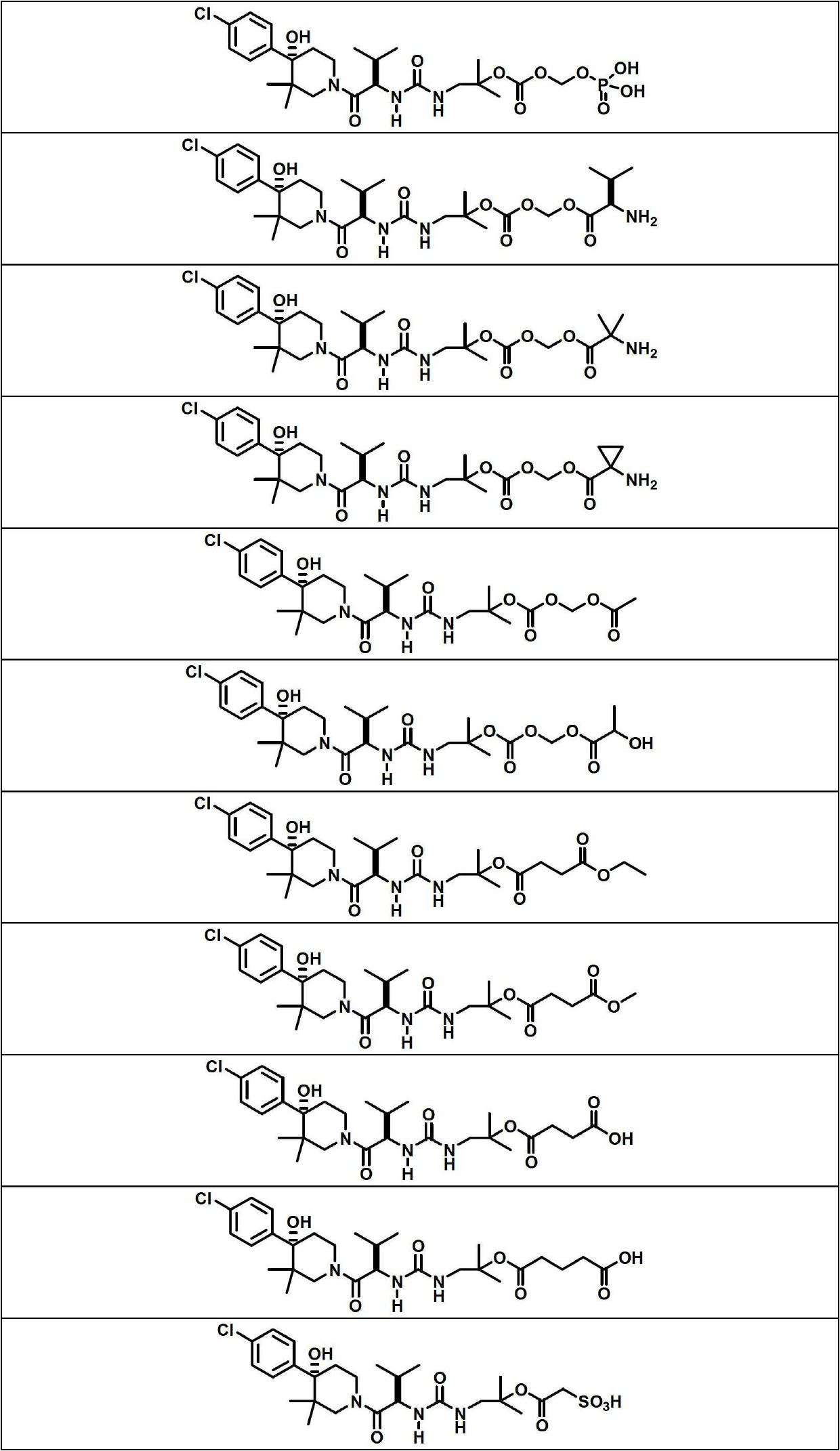
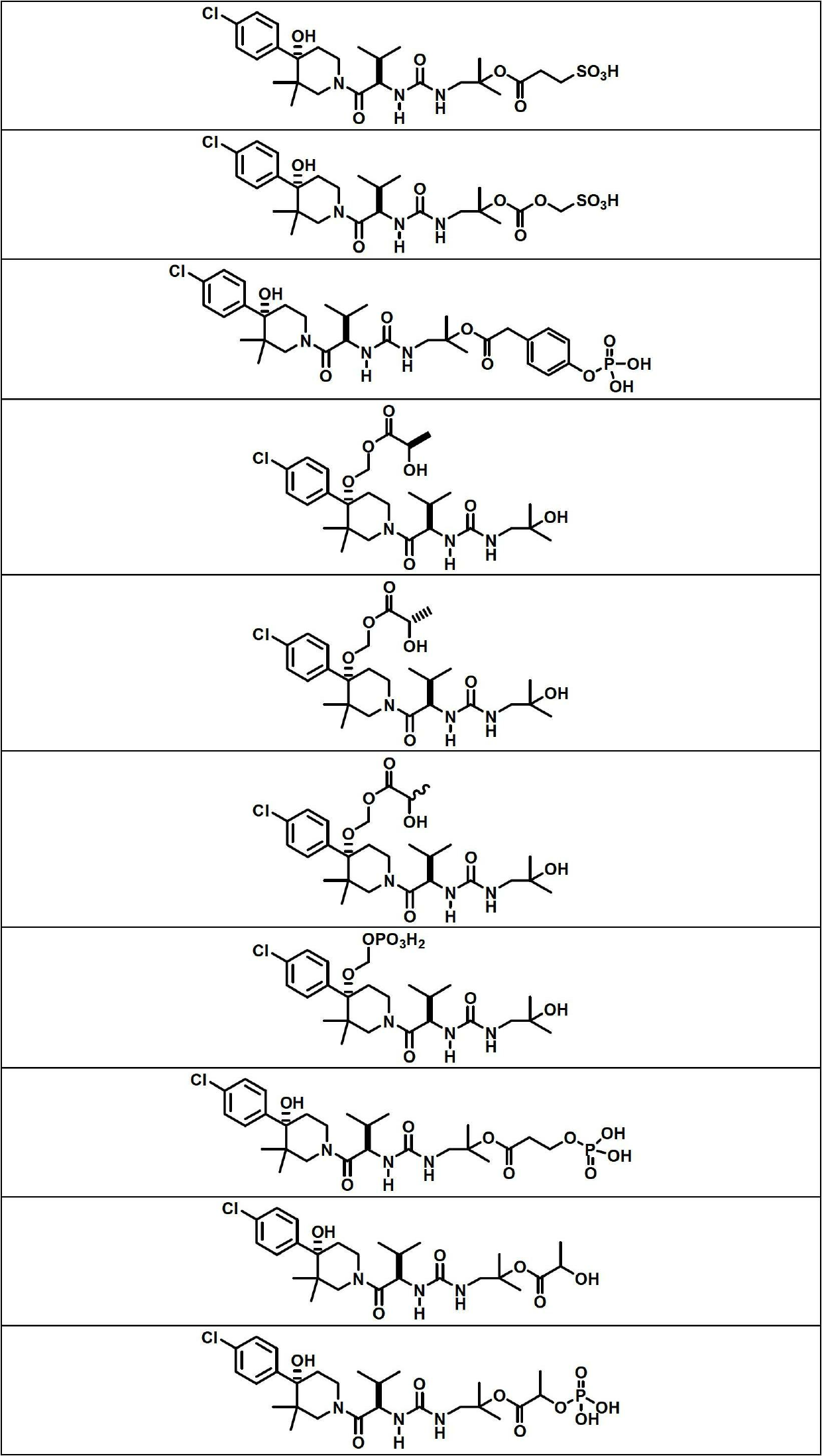
Image
Examples
Embodiment 1
[0108] Step 1: tert-Butyl 3,3-Dimethyl-4-oxopiperidine-1-carboxylate
[0109]
[0110] A solution of tert-butyl 4-oxopiperidine-1-carboxylate (52.47 g, 263 mmol) in THF (1000 mL) was cooled to 0 °C and treated with sodium hydride (60% in mineral oil) at 5 min intervals suspension) (22.12 g, 553 mmol) was processed in 4 equal portions. The resulting suspension was stirred at 0 °C for 45 minutes ("min."), and then treated by dropwise addition of iodomethane (41.2 ml, 658 mmol). The mixture was stirred for 1 hour ("h" or "hr"), and then allowed to come to room temperature ("rt"). Ninety minutes after the ice bath was removed, a rapid exotherm (20-40° C. within 3 minutes) and vigorous evolution of gas was observed. The ice bath was changed, and the mixture was stirred overnight, at which time the mixture was slowly warmed to room temperature. The reaction mixture was quenched with saturated ammonium chloride (200 mL), then treated with sufficient water to dissolve the precip...
Embodiment 2
[0139]
[0140] Compound 1 (200 mg, 0.396 mmol), BOC-L-alanine (188 mg, 0.991 mmol) were treated with a solution of N,N'-dicyclohexylcarbodiimide (229 mg, 1.110 mmol) in DCM (2 mL) . , a solution of 4-(dimethylamino)pyridine (121 mg, 0.991 mmol) in DCM (5 mL), and the mixture was stirred at room temperature for 24 hours. The reaction mixture was filtered to remove N,N-dicyclohexylurea and the filtrate was concentrated on a rotary evaporator. The residue was dissolved in EtOAc (15 mL), cooled to 0 °C and additional N,N-dicyclohexylurea was filtered off. Use 1M KHSO 4 (3 times), water and 5% NaHCO 3 (3 times) wash filtrate, dry (Na 2 SO 4 ) and concentrate. Purification of the crude product by flash chromatography using a 12 g silica gel column and eluting with 0-75% EtOAc / hexanes afforded (S)-2-(tert-butoxycarbonylamino)propanoic acid 1-(3-((R )-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methyl-1-oxobutane-2 -yl)ureido)-2-methylpropan-2-yl ester...
Embodiment 3
[0147]
[0148] Compound 1 (215 mg, 0.426 mmol), BOC-L-valine (231 mg, 1.066 mmol) were treated with a solution of N,N'-dicyclohexylcarbodiimide (246 mg, 1.193 mmol) in DCM (2 mL) and 4-(dimethylamino)pyridine (130 mg, 1.066 mmol) in DCM (5 mL), and the mixture was stirred at room temperature for 48 hours. The reaction mixture was filtered to remove N,N-dicyclohexylurea and the filtrate was concentrated on a rotary evaporator. The residue was dissolved in EtOAc (15 mL), cooled to 0 °C and additional N,N-dicyclohexylurea was filtered off. Use 1M KHSO 4 (3 times), water and 5% NaHCO 3 (3 times) wash filtrate, dry (Na 2 SO 4) and concentrate. Purification of the crude product by flash chromatography using a 12 g silica gel column and eluting with 0-75% EtOAc / hexanes afforded the desired product (S)-2-(tert-butoxycarbonylamino)-3-methylbutanoic acid 1 -(3-((R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methyl- 1-oxobut-2-yl)ureido)-2-methylpropan-2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com