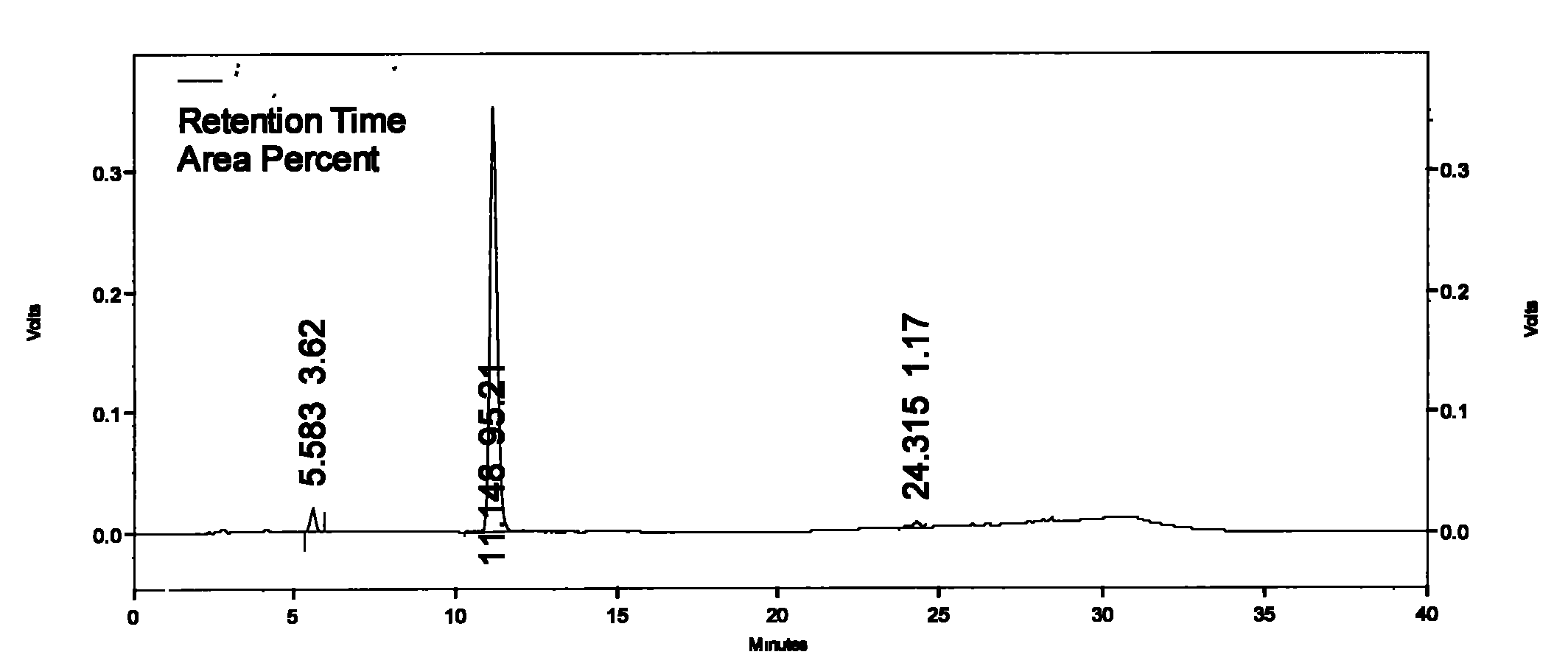
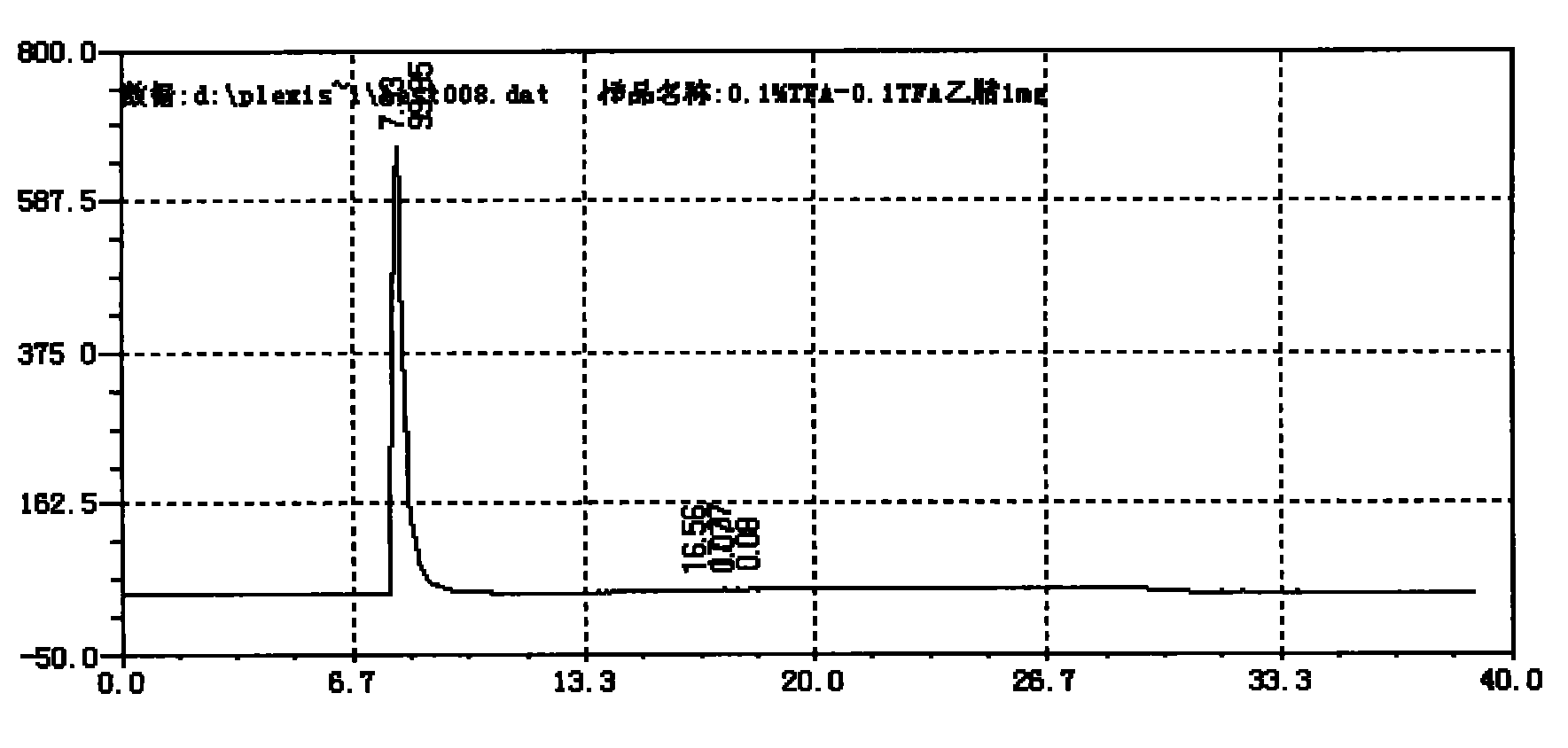
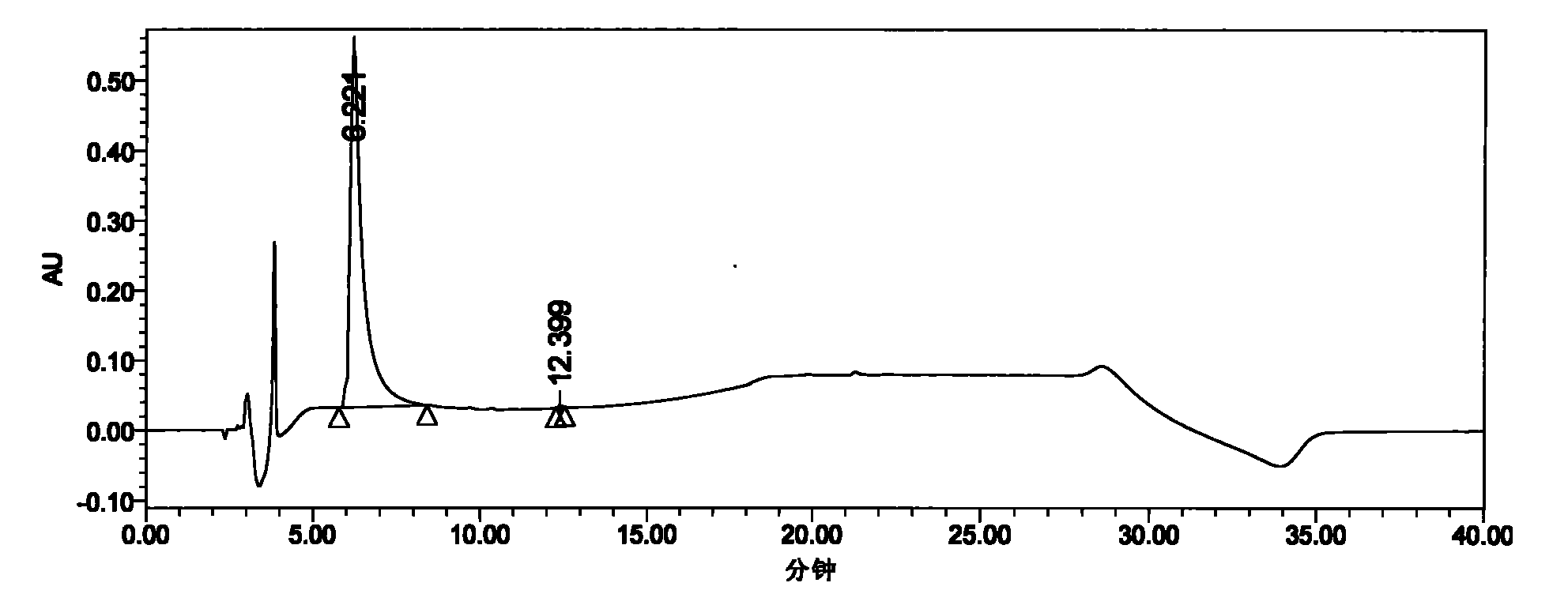
Preparation method of Plerixafor
A Plerixafor and structural formula technology, applied in the direction of organic chemistry, can solve problems such as difficult removal, incomplete reaction, and reduced activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Implementation Example 1: Preparation of 3Boc-protected 1,4,8,11-tetraazacyclotetradecane
[0048] Take 10g (0.05mol) of 1,4,8,11-tetraazacyclotetradecane, add 50ml of acetone-water (2:1), add 10.119g (0.1mol) of triethylamine, diisopropylethylamine 3.225 g (0.025 mol), 38.194 g (0.175 mol) of di-tert-butyl carbonate was added dropwise at room temperature, stirred at room temperature for 24 hours after the dropwise addition, and the reaction was monitored by HPLC. After the reaction was completed, the reaction was evaporated to dryness at 50°C under reduced pressure to obtain a light yellow oil, which was applied to a 150 g silica gel column and eluted with ethyl acetate. The ethyl acetate solution was collected and evaporated to dryness under reduced pressure to obtain 23.12 g of a white foam. 92.36%. 1 HNMR (400MHz, CDCl 3 , δppm): 1.74 (2H, q, 5.5); 1.96 (2H, q, 6.5); 2.66 (2H, t, 5.5); 2.82 (2H, t, 5.5); 3.33 (4H, m); , m); 3.37 (2H, m), 3.43 (4H, m).
Embodiment 2
[0049] Implementation Example 2: Preparation of 6Boc-protected Plerixafor
[0050]Take 20.03g (0.04mol) of 3Boc-protected 1,4,8,11-tetraazacyclotetradecane, dissolve it in 400ml of anhydrous acetonitrile, add 20g of anhydrous potassium carbonate, 3.5012g of α-α'dichloroxylene (0.02mol), sodium iodide 75mg, refluxed under nitrogen protection for 24 hours, and TLC monitored the reaction. After completion of the reaction, let cool to room temperature, filter with suction, wash the filter cake with 200ml of acetonitrile, combine the acetonitrile solution, and evaporate to dryness under reduced pressure to obtain 21.20 g of 6Boc-protected plerixafor with a yield of 96.06%. Recrystallized with ethanol-water mixed solvent to obtain a white solid.
Embodiment 3
[0051] Implementation Example 3: Plerixafor·8HCl·3H 2 O compound preparation
[0052] Take 20 g of 6Boc-protected plerixafor, add 200 ml of methanol, stir to dissolve, add 60 ml of concentrated hydrochloric acid dropwise at room temperature, stir at room temperature for 48 hours after the dropwise addition, and monitor the reaction by TLC. After the reaction was completed, it was filtered with suction, and the filter cake was dried under reduced pressure at 50° C. to obtain 13.54 g of white solid, with a yield of 88.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com