Application of pyrilamine compounds to preparation of acetylcholinesterase inhibitor
A technology of acetylcholinesterase and pyrimidine amines, which is applied in the field of pyrimidine amine compounds and can solve the problems that there are no pyrimidine amine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
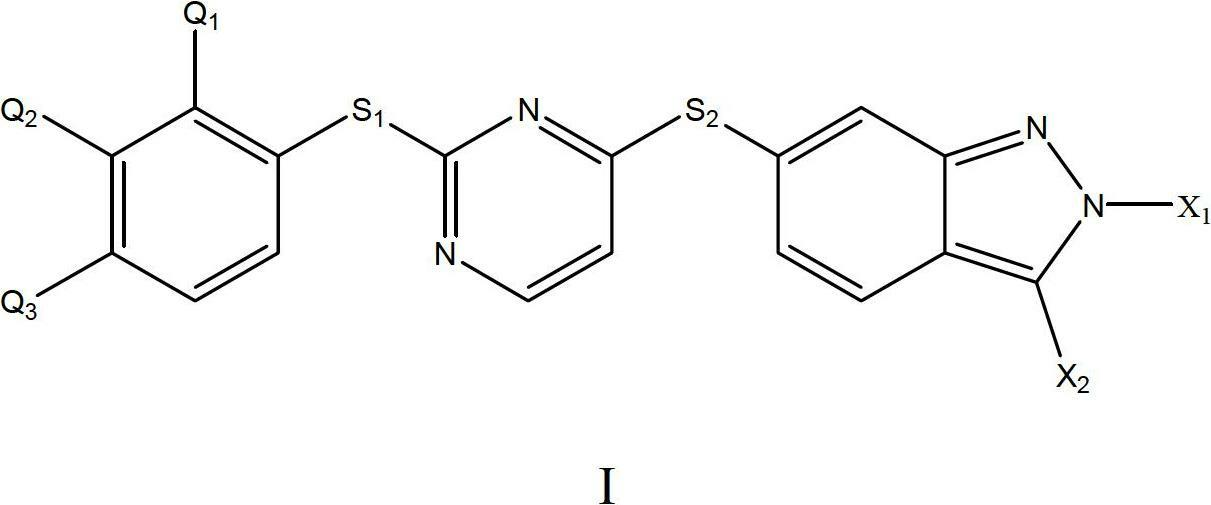
[0039]The preparation of the acetylcholinesterase inhibitor of the present invention includes combining the compound of formula I described in the present invention, or its pharmaceutically acceptable salt and solvate thereof, with a common pharmaceutical adjuvant or carrier to prepare a compound having acetylcholinesterase Pharmaceutical compositions with enzyme inhibitory activity. The above-mentioned pharmaceutical composition can adopt dosage forms such as tablets, granules, capsules, oral liquids, injections, and aerosols; it can also adopt controlled-release or sustained-release dosage forms or nano-preparations known in the modern pharmaceutical industry. Regarding the selection of preparation methods and methods, it is believed that those skilled in the art can obtain sufficient facts from the prior art, and the present invention will not repeat them.
[0040] Following non-restrictive embodiment can make those of ordinary skill in the art understand the present invent...
Embodiment
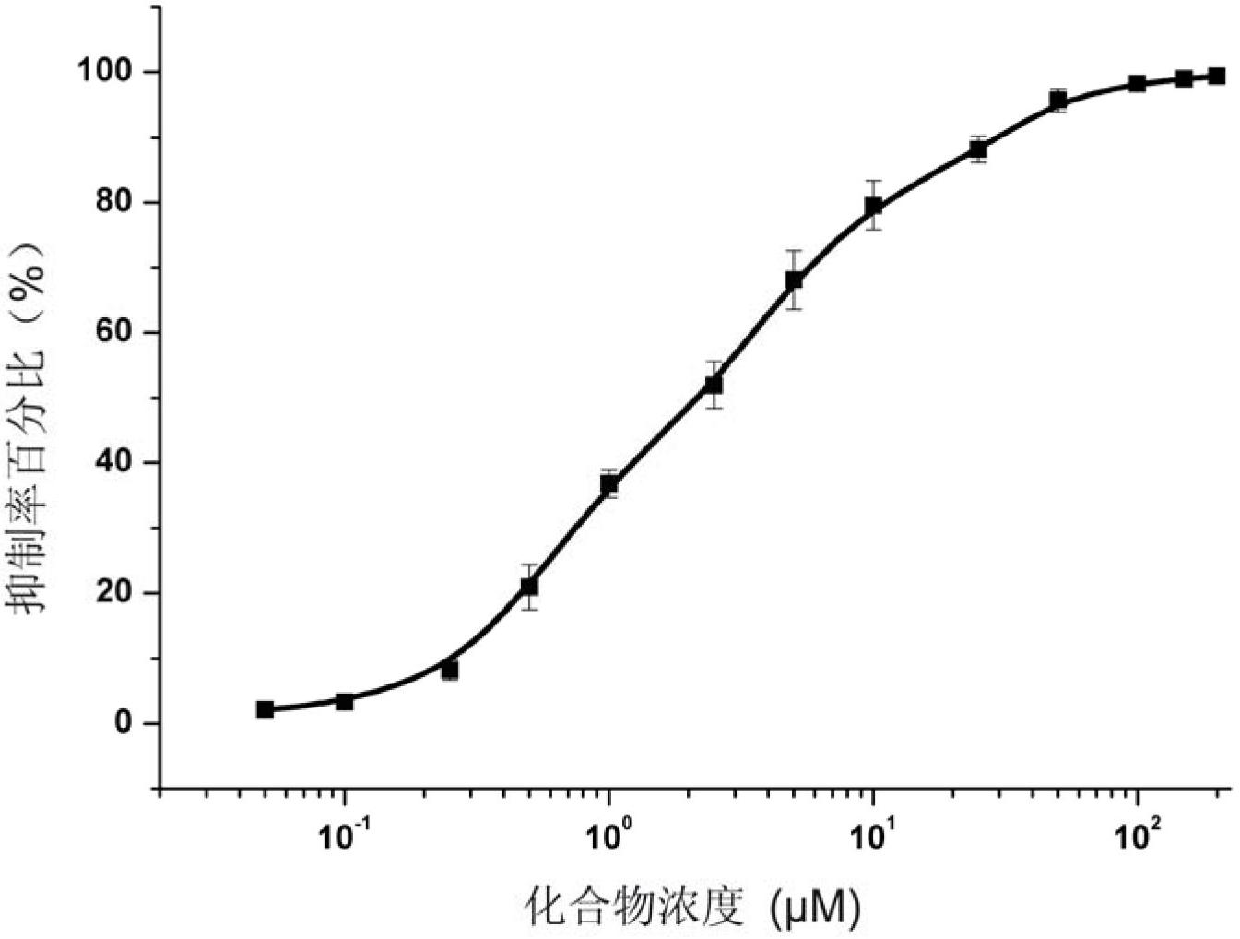
[0042] Pazopanib (compound 1) inhibits acetylcholinesterase activity detection
[0043] The pyrimidine amine compound pazopanib (compound 1) used in the test of the present invention was prepared according to the method reported in the literature: US 2009 / 0005406A1 (disclosure date: January 1, 2009).
[0044]
[0045] Inhibition of acetylcholinesterase activity detection test:
[0046] Fresh acetylcholinesterase, purchased from Sigma company;
[0047] Thioacetylcholine salt (ATOH) was purchased from Sigma as a substrate;
[0048] 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) is a chromogen, purchased from Sigma;
[0049] 0.01M phosphate buffer (pH=7.0);
[0050] Pazopanib was made into DMSO solution (both 20 μmol / mL).
[0051] Detection method:
[0052] (1) Acetylcholinesterase and substrate are prepared with 0.01M phosphate buffer solution at a suitable concentration. 1ml of the initial reaction system contains 0.02 units of enzyme, 0.1 μmol of substrate, 0.1 μmol of chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com