Catalyst for synthesizing ethylene amine and method for preparing ethylene amine
A technology of catalyst and ethyleneamine, which is applied in the field of catalyst for the synthesis of ethyleneamine, which can solve the problems of severe equipment corrosion, high production cost, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
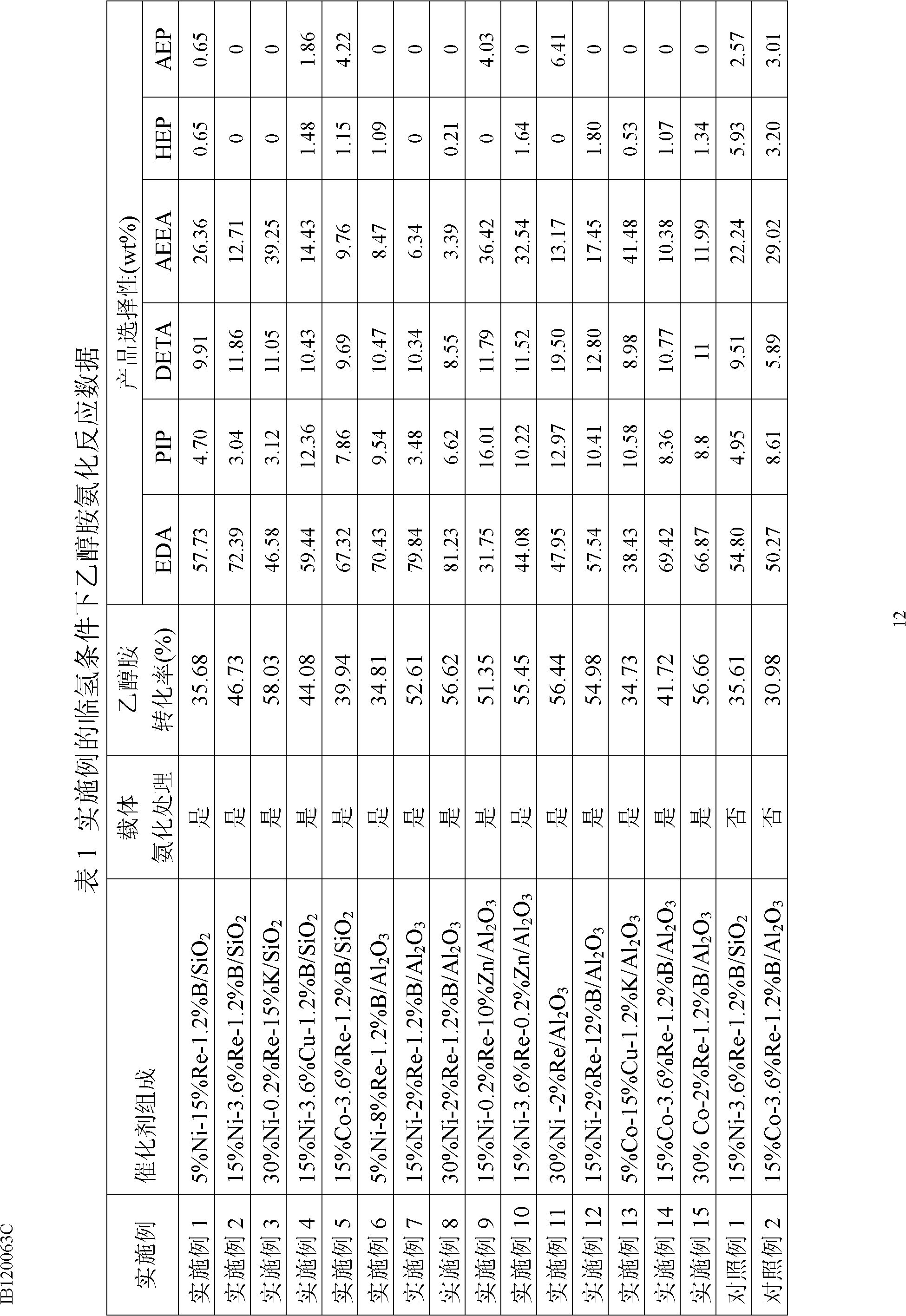
Embodiment 1
[0033] 5%Ni-15%Re-1.2%B / SiO 2 Catalyst preparation and application
[0034] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Install in a quartz tube, dry at 200°C for 5 hours under an inert atmosphere, then introduce 10% ammonia-hydrogen gas mixture (molar content), ammoniation temperature is 300°C, and ammoniation time is 5 hours. 2.477 g Ni(NO 3 ) 2 ·6H 2 O, 2.161 g NH 4 ReO 4 and 0.686 g H 3 BO 3 Dissolve in 12ml deionized water. Half of this aqueous solution was used to impregnate the above ammoniated SiO 2 The carrier was air-dried, then dried at 120°C for 4 hours, and then calcined at 500°C for 4 hours. Then, impregnate the ammonified SiO 2 The carrier is then air-dried, dried at 120°C for 4 hours, and calcined at 500°C for 4 hours. Before the catalyst is used, in a hydrogen flow at 375°C (atmospheric pressure, 2000h -1 ) to reduce for 4 hours. When the temperature in the reactor is naturally lowered to 160°C, the pressure is increased to 8MPa. Af...
Embodiment 2
[0036] 15%Ni-3.6%Re-1.2%B / SiO 2 Catalyst preparation and application
[0037] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Install in a quartz tube, dry at 200°C for 5 hours under an inert atmosphere, then introduce 20% ammonia-hydrogen gas mixture (molar content), ammoniation temperature is 300°C, and ammoniation time is 5 hours. 7.432 g Ni(NO 3 ) 2 ·6H 2 O, 0.518 g NH 4 ReO 4 and 0.686 g H 3 BO 3 Dissolve in 12ml deionized water. Refer to Example 1 for the remaining preparation steps and catalyst evaluation scheme. The reaction results are shown in Table 1.
Embodiment 3
[0039] 30%Ni-0.2%Re-15%K / SiO 2 Catalyst preparation and application
[0040] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Install in a quartz tube, dry at 200°C for 5 hours under an inert atmosphere, then introduce 50% ammonia-hydrogen gas mixture (molar content), ammoniation temperature is 300°C, and ammoniation time is 5 hours. 14.864 g Ni(NO 3 ) 2 ·6H 2 O, 0.029 g NH 4 ReO 4 and 3.879 grams of KNO 3 Dissolve in 12ml deionized water. Refer to Example 1 for the remaining preparation steps and catalyst evaluation scheme. The reaction results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com