New application of traditional Chinese medicine fennel
A technology of cumin and traditional Chinese medicine, which is applied to the new application field of traditional Chinese medicine cumin, can solve the problems of low efficiency, lack of practical value, inability to effectively overcome the natural barrier effect of the intestinal tract and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of fennel sausage tract absorption enhancer decoction: The traditional Chinese medicine fennel was decocted according to the conventional method to obtain 0.3g / ml (dry weight / liquid) decoction, and the gavage dose was 0.5ml decoction / head Mice.
Embodiment 2
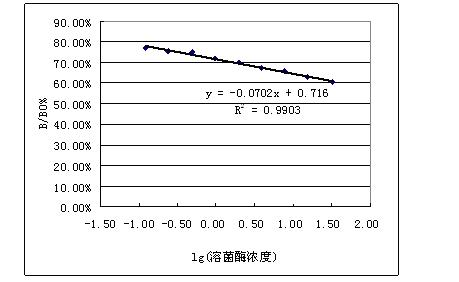
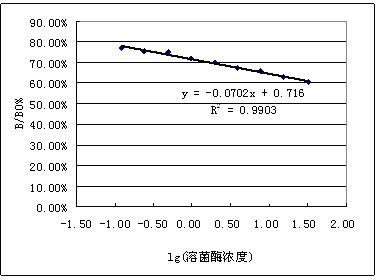
[0018] Example 2: Preparation of lysozyme sodium alginate microspheres: prepared by W / O emulsification-ion crosslinking method according to literature reference. The main preparation parameters are: sodium alginate concentration 5% (W / V), lysozyme concentration 1% (W / V), fructose concentration 2% (W / V). Properties of drug-loaded microspheres: spherical shape, smooth and round surface, less adhesion, no damage, good dispersion and stability, average particle size (9.45±3.1) μm, encapsulation efficiency 57.6%, drug loading It was 7.2%, and the enzyme activity was 76.1% of the initial lysozyme activity.
Embodiment 3
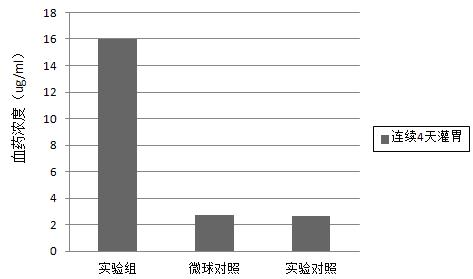
[0019] Example 3: Oral mode and dosage: mice were simultaneously gavage with fennel decoction and drug-loaded sodium alginate microspheres, once a day, the dosage of fennel decoction was 0.5 ml per mouse. The dose of drug-loaded sodium alginate microspheres is 14 mg / mouse.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com