Novel application of rutaecarpine compound
A technology of compound and application, which is applied in the field of new application of rutaecarpine compounds, can solve problems such as incurability, achieve good anti-atherosclerosis effect, and have broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
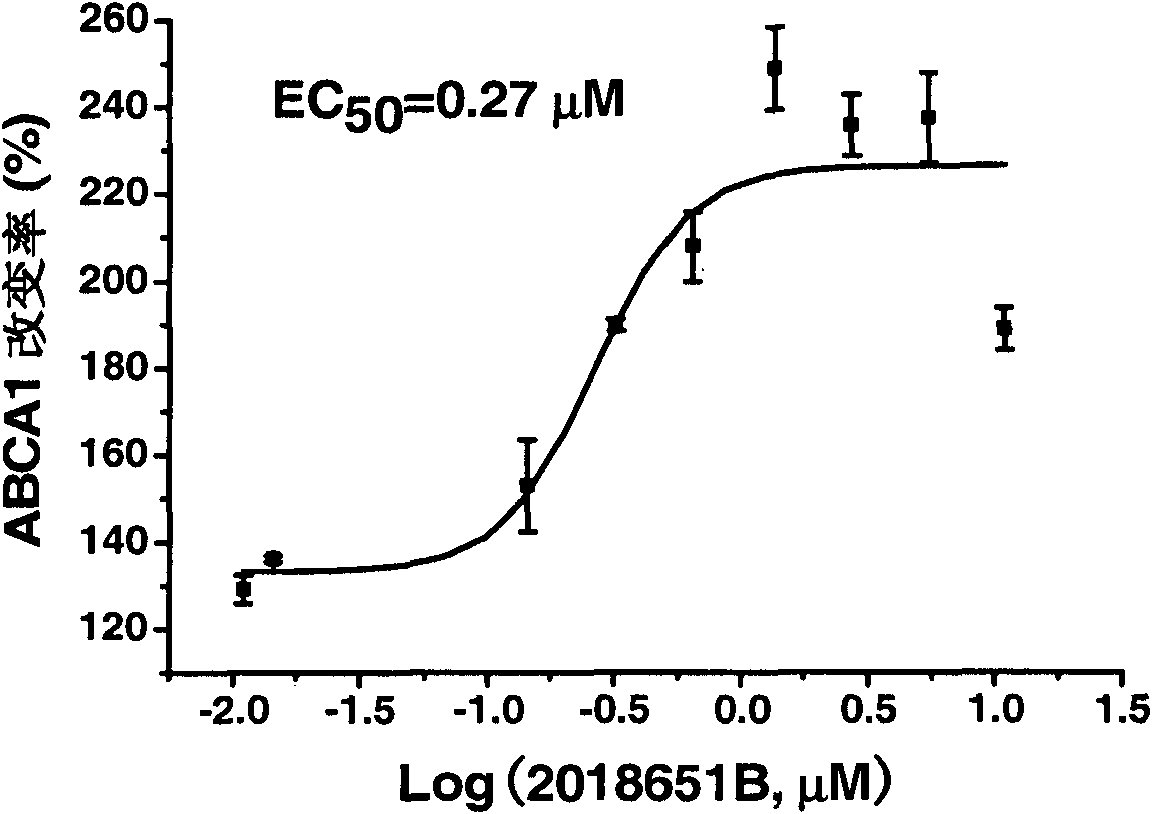
[0043] Example 1: Activity of evodiamine on ABCA1 up-regulation screening model
[0044] ABCA1 up-regulator screening model principle: Insert the upstream regulatory sequence of ABCA1 (ref|NT_008470.18|Hs9_8627, -819bp~+67bp) into the upstream of the luciferase reporter gene of pGL3-BasicVector (Promega Company) to obtain the recombinant plasmid pGL3-ABCA1. pGL3-ABCA1 and pcDNA3 were co-transfected into human liver cancer cell HepG2, and a stable transfected cell line was obtained by G418 screening, which was named ABCA1-LUC HepG2 cells. The up-regulation activity of the drug on receptor gene expression was indirectly reflected by measuring the change of its fluorescence intensity. .
[0045]Determination of cell luciferase expression activity: Luciferase expression activity was determined using a luciferase detection kit Luciferase Assay System (Promega). The bioluminescent reaction catalyzed by firefly luciferase is as follows:
[0046]
[0047] ABCA1-LUC HepG2 cells in...
Embodiment 2
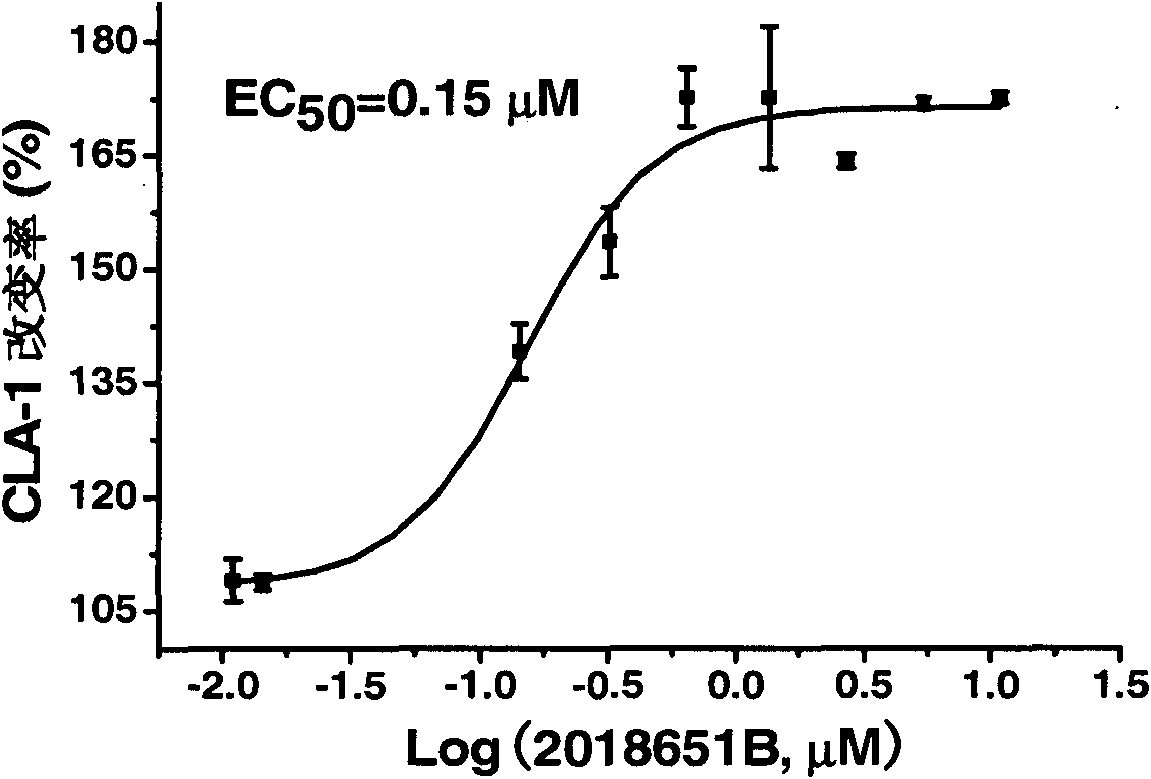
[0056] Example 2: Activity of evodiamine on CLA1 up-regulation screening model
[0057] CLAP-LUC HepG2 cells (invention patent 200410029902.4, human high-density lipoprotein receptor expression up-regulator screening model) were mixed with 5×10 4 Each cell / well was seeded in a 96-well cell culture plate. After the cells had adhered to the wall for about 6 hours, the medium was replaced with 200 μl / well of serum-free MEM containing a series of diluted concentrations of evodiamine and evodiamine- EBSS medium (Hyclone; because the people who built the model used two different mediums, it has been continued; because CLA1 is also involved in reverse cholesterol transport (RCT), up-regulating CLA1 and ABCA1 will be beneficial against AS, and the regulation of ABCA1 sequences have partially identical regulatory elements; both compounds are dosed in parallel). Note that the final concentration of DMSO in each concentration well is kept at 0.1% (and added at the same time as the drug ...
Embodiment 3
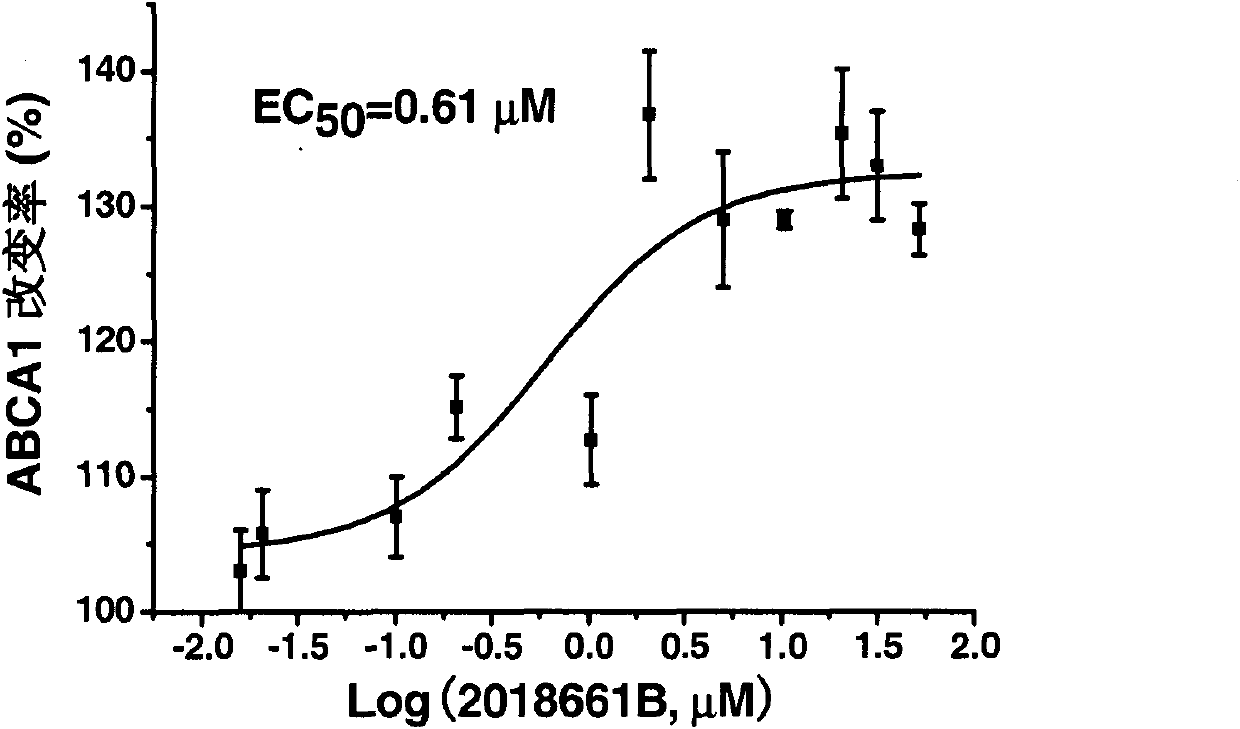
[0067] Example 3: Agonist activity of evodiamine on PPARγ
[0068] A) Effects of Compounds on PPARγ Transcriptional Activation
[0069] The nuclear receptor PPARγ has two main domains: ligand-binding domain (LBD) and DNA-binding domain (DBD), each of which has independent functions. Using the PPARγ agonist model constructed by our laboratory (Patent No. 200710061911.5, new xanone compounds and their preparation methods and uses), the ligand-binding domain (LBD) of PPARγ and the DNA-binding domain of the yeast cell transcription factor GAL4 (DBD) was constructed as a fusion expression vector pBIND-PPARγ-LBD. The corresponding elements of 5×GAL4 were artificially synthesized and inserted into the upstream of the reporter gene to construct the reporter plasmid pG5-promotor-GAL4 (GAL4 for short).
[0070] The GAL4 reporter plasmid and the constructed recombinant plasmid pBIND-PPARγ-LBD were subjected to Lipofectamine TM 2000 (Invitrogen) mediated co-transfection of HepG2 cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com