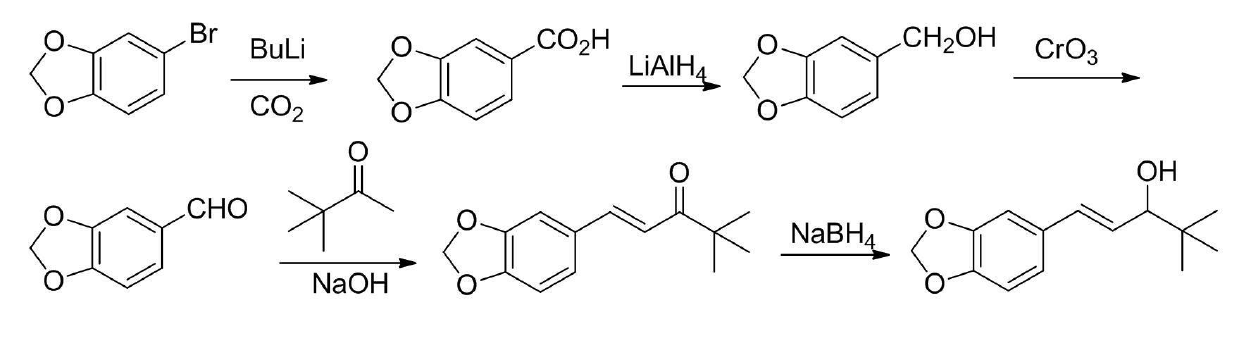
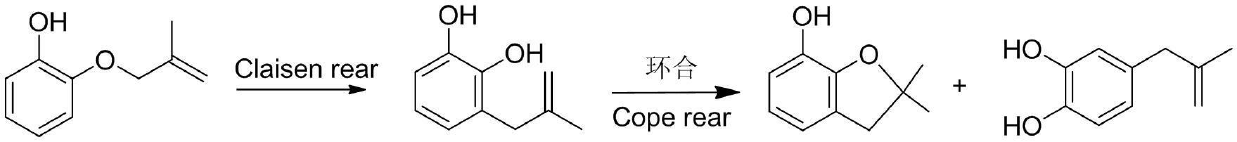
Preparation method of stiripentol
A technology of stiripentol and methallyl, which is applied in the field of preparation of epilepsy drugs, can solve the problems of difficulty in determining the frequency of epileptic seizures, increase blood concentration, etc., and achieves the effects of easy separation and prevention of disproportionation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
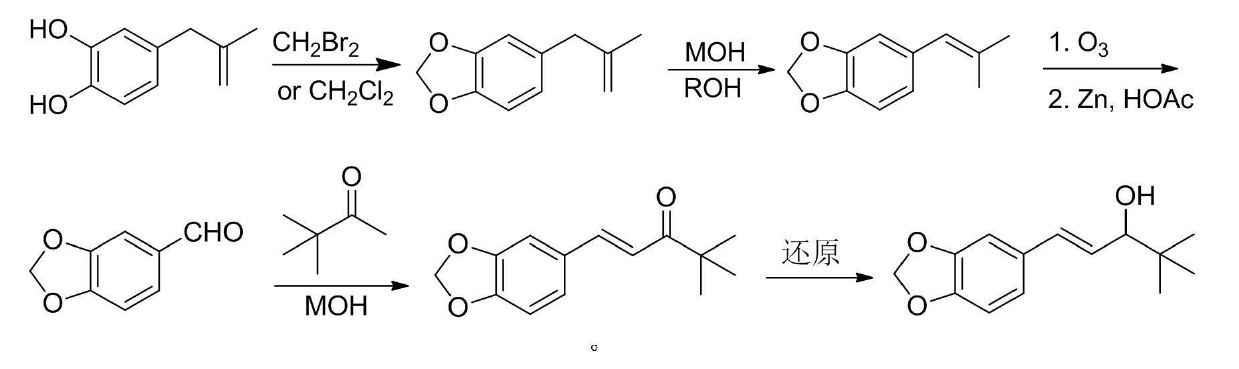
[0031] Preparation of 5-(2-methallyl)benzo[d][1,3]dioxole
[0032]
[0033] 30mL of water, 26.1g of dibromomethane, stirred and heated to reflux. Slowly add 16.4g of 4-(2-methylallyl)-1,2-benzenediol, 10.0g of sodium hydroxide and 50mL of water mixture, after the addition is complete, continue the reflux reaction for 2h; cool, add 100mL of ethyl acetate, Layered, washed with a small amount of aqueous sodium hydroxide solution, washed with water (2×100mL); the organic phase was dried with anhydrous sodium sulfate, spin-dried to dry the solvent, and distilled to obtain 12.9g of colorless liquid 5-(2-methallyl)benzo [d][1,3]dioxole, yield 73%, 1 HNMR (CDCl 3 , 300MHz), δ: 1.67(t, J=1.2Hz, 3H, CH 3 ), 3.23 (s, 2H, CH 2 ), 4.73(q, J=1.2Hz, 1H, C=CH), 4.79~4.80(q, J=1.2Hz, 1H, C=CH), 5.93(s, 2H, CH 2 O), 6.63 ~ 6.75 (m, 3H, C 6 h 3 ).GC-MS (m / z): 176 (M + ), 151, 131, 103, 77.
Embodiment 2
[0035] Preparation of 5-(2-methylpropenyl)benzo[d][1,3]dioxole
[0036]
[0037] 21.1g 5-(2-methallyl)benzo[d][1,3]dioxole, 4.2g KOH and 25mL n-butanol, react at 80°C for 6h; In ice water, add dilute hydrochloric acid dropwise until neutral, add 50mL of ethyl acetate to extract, wash with water, dry, and recover the solvent to obtain 18.4g of light yellow liquid 5-(2-methylpropenyl)benzo[d][1,3 ] Dioxole, yield 87%.
Embodiment 3
[0039] Preparation of piperonal
[0040]
[0041] 4.2g (0.024mol) 5-(2-methylpropenyl)benzo[d][1,3]dioxole and 60mL acetic acid, cooled to 0°C in an ice-salt bath, and passed through O 3 , oxidized for 1.0h, TLC monitors that the raw material point disappears; 2 1.0h, slowly add 10.0g Zn powder, continue stirring at 0°C for 2.0h, filter, extract with 20mL ethyl acetate, wash with water, and dry over anhydrous sodium sulfate. Column chromatography gave 3.1 g of a colorless liquid, which was placed in a refrigerator to give a colorless crystal of piperonal with a yield of 74% and a melting point of 36.8-37.7°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com