2,6-diisopropyl aniline salicylaldiminato nickel, and preparation and application thereof
A technology of diisopropylaniline and propylaniline water, which is applied to 2,6-diisopropylaniline salicylic aldimine nickel and its preparation and application fields, can solve the problems of high cost, difficult to obtain raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
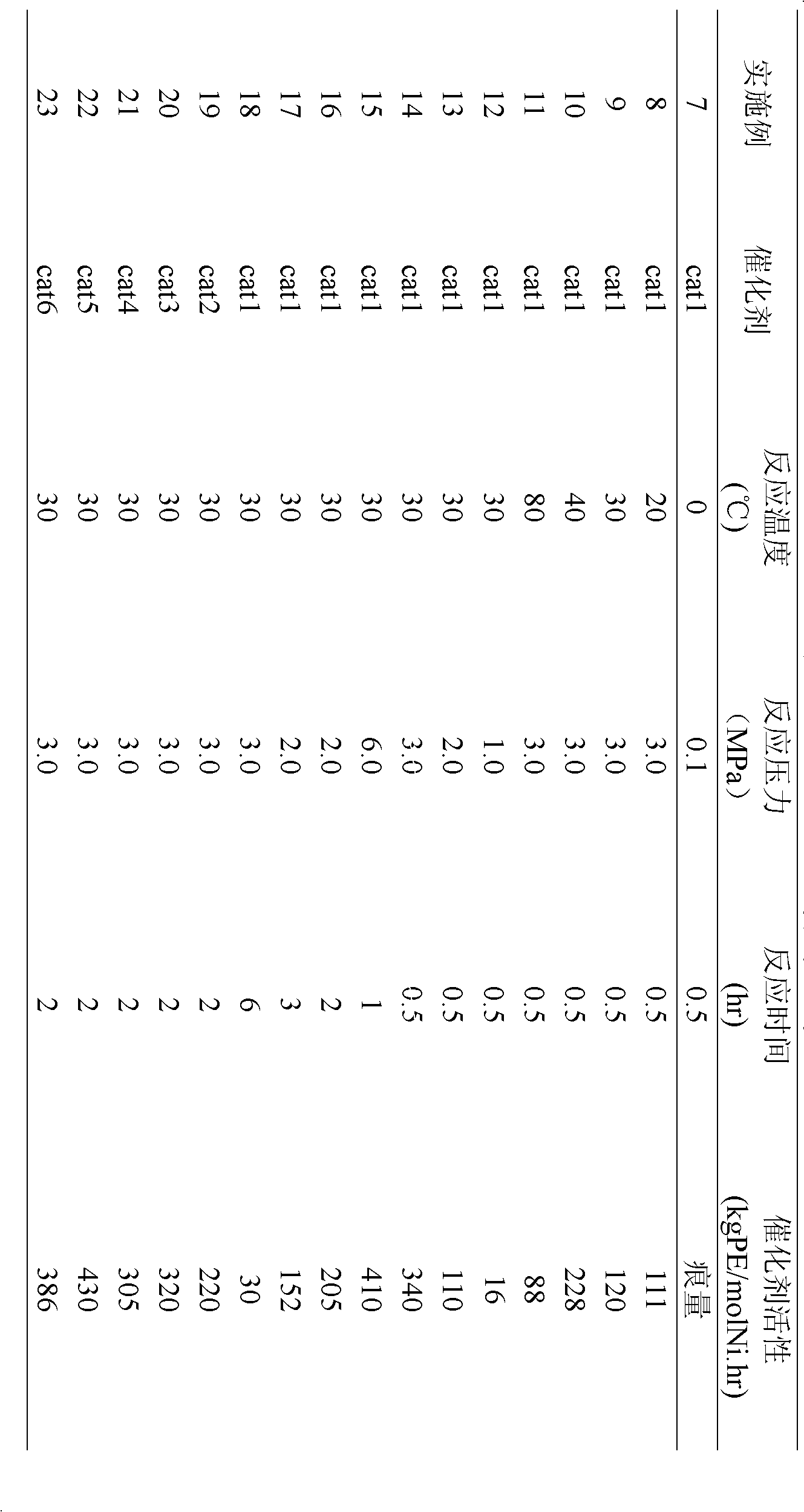
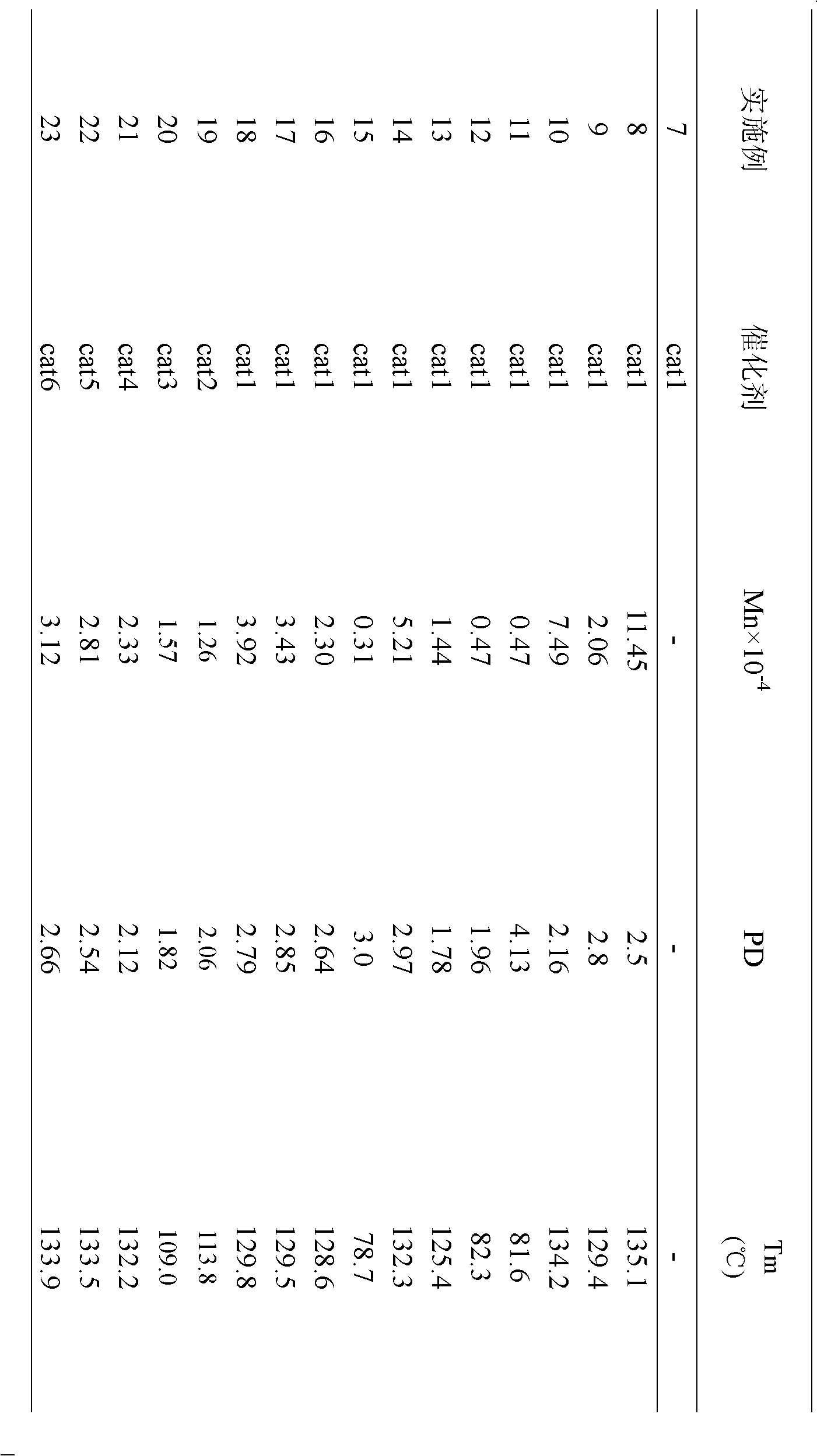
Examples
Embodiment 1
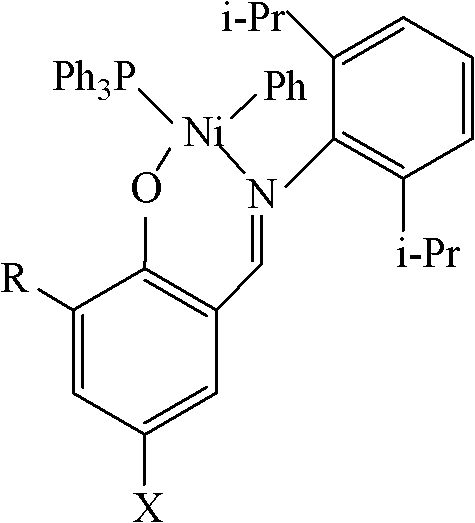
[0016] [O-(3-CH 3 )C 6 h 3 CH=N-2,6-C 6 h 3 i PR 2 ]Ni(PPh 3 )(Ph)(cat1) Preparation
[0017] Under the protection of an inert gas, 3-methyl salicylaldehyde (2.72g, 0.02mol) was dissolved in 100mL ethanol solvent, 2,6-diisopropylaniline (3.8mL, 0.02mol) was added, and in 0.5mL acetic acid Under the action, react at 0°C for 12 hours, and naturally cool to room temperature to obtain light yellow needle-like solid, namely 3-methyl-salicylaldimine ligand. Dissolve 1.6mmol of this ligand in 80mL of tetrahydrofuran, add NaH (38mg, 1.6mmol), react at -20°C for 6 hours, and transfer the supernatant to the metal nickel compound trans-[NiCl(Ph)(PPh 3 ) 2 ] (1.1g, 1.6mmol) in toluene solvent, react at -20°C for 48 hours. After the reaction, the solvent was drained, and the residue was recrystallized with an organic solvent composed of toluene and n-hexane (volume ratio: 1:1) to obtain 10.68 g of a solid catalyst cat with a yield of 62%.
Embodiment 2
[0019] [O-(3-iC 3 h 7 )C 6 h 3 CH=N-2,6-C 6 h 3 i PR 2 ]Ni(PPh 3 )(Ph)(cat2) Preparation
[0020] Under the protection of an inert gas, 3-isopropyl salicylaldehyde (3.28g, 0.02mol) was dissolved in 50mL ethanol solvent, 2,6-diisopropylaniline (4.3mL, 0.023mol) was added, and in 0.5mL Under the action of acetic acid, reacted at 20°C for 10 hours, and naturally cooled to room temperature to obtain light yellow needle-like solid, namely 3-isopropyl-salicylaldimine ligand. Dissolve 2.4mmol of this ligand in 80mL of tetrahydrofuran, add NaH (76mg, 3.2mmol), react at -10°C for 4 hours, and transfer the supernatant to the metal nickel compound trans-[NiCl(Ph)(PPh 3 ) 2 ] (1.1g, 1.6mmol) in toluene solvent, react at -10°C for 36 hours. After the reaction, the solvent was drained, and the residue was recrystallized with an organic solvent composed of toluene and n-hexane (volume ratio: 2:1) to obtain 20.60 g of a solid catalyst cat with a yield of 52%.
Embodiment 3
[0022] [O-(3-CH 3 )-(5-Br)C 6 h 3 CH=N-2,6-C 6 h 3 i PR 2 ]Ni(PPh 3 )(Ph)(cat3) Preparation
[0023] Under the protection of an inert gas, 3-methyl-5-bromosalicylaldehyde (4.28g, 0.02mol) was dissolved in 50mL ethanol solvent, 2,6-diisopropylaniline (4.5mL, 0.024mol) was added, Under the action of 0.5 mL of acetic acid, reacted at 40°C for 8 hours, and naturally cooled to room temperature to obtain a light yellow needle-like solid, namely 3-methyl-5-bromosalicylaldimine ligand. Dissolve 3.2 mmol of this ligand in 80 mL of tetrahydrofuran, add NaH (115 mg, 4.8 mmol), react at -5°C for 3 hours, and transfer the supernatant to the metal nickel compound trans-[NiCl(Ph)(PPh 3 ) 2 ] (1.1g, 1.6mmol) in toluene solvent, react at 0°C for 24 hours. After the reaction, the solvent was drained, and the residue was recrystallized with an organic solvent composed of toluene and n-hexane (volume ratio: 4:1) to obtain 30.86 g of solid catalyst cat with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com