Preparation method of styrene-acrylic resin with high solid content
A high solid content, styrene-acrylic resin technology, applied in the application field of branched polymers in polymer synthesis, can solve the problems of insufficient hydroxyl number, incomplete cross-linking and curing, affecting resin performance, etc. The effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
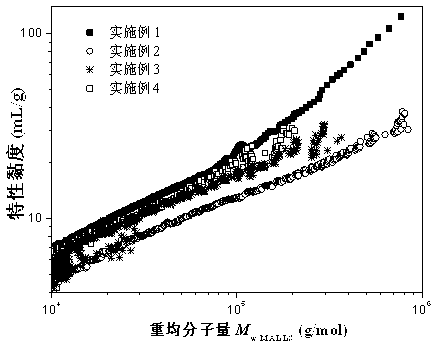
[0018] The monomers styrene (5.2365g, 0.05mol), methyl methacrylate (5.1993g, 0.052mol), n-butyl acrylate (7.4008g, 0.057mol), isooctyl acrylate (3.6563g, 0.020mol), Hydroxypropyl acrylate (9.0095g, 0.069mol), functional monomer dimer (0.4123g), initiator BPO (0.5124g, 2mmol) were mixed evenly, and added dropwise to the solvent xylene (7.6874g ), propylene glycol methyl ether acetate (2.7048g) and heavy aromatics (2.7105g), the system was refluxed for 4 hours, and the mixture was added dropwise. The reaction was continued at constant temperature for 3 hours, and then the reaction was terminated to obtain a colorless and transparent resin. The solid content of the resin was measured to be 70.22%, and the viscosity measured by the Grignard tube was 140.9s. Polymer is analyzed by three-detection gel permeation chromatography, and the results are as follows: light scattering weight average molecular weight M w.MALLS =22260, molecular weight distribution PDI=2.0. figure 1 is the...
Embodiment 2
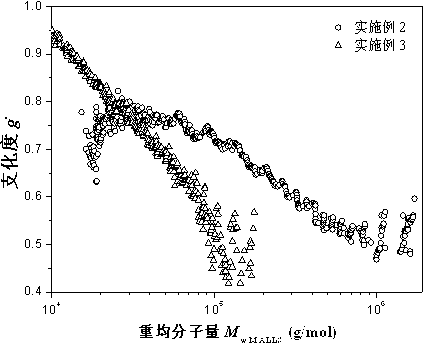
[0020] The monomers styrene (5.2146g, 0.05mol), methyl methacrylate (5.2017g, 0.052mol), n-butyl acrylate (10.4662g, 0.081mol), hydroxypropyl acrylate (9.0020g, 0.069mol), The functional monomer 3-mercaptopropionyloxyethyl methacrylate (0.2256g, 0.52mmol) and the dimer (0.1864g), the initiator BPO (0.0230g, 0.09mmol) were mixed evenly, and added dropwise to temperature In the solvent xylene (7.6795g), propylene glycol methyl ether acetate (2.6872g) and heavy aromatics (2.6898g) at 135°C, the system was refluxed for 4 hours, and the mixture was added dropwise. The reaction was continued at a constant temperature for 3 hours, and the reaction was terminated to obtain a slightly yellowish transparent resin. The measured resin solid content was 68.51%, and the Grignard tube measured its viscosity as 131.3s. Polymer is analyzed by three-detection gel permeation chromatography, and the results are as follows: light scattering weight average molecular weight M w.MALLS =122300, mole...
Embodiment 3
[0022] The monomers styrene (5.1993 g, 0.05 mol), methyl methacrylate (5.2088 g, 0.052 mol), n-butyl acrylate (9.4562 g, 0.073 mol) and hydroxypropyl acrylate (8.0192 g, 0.062 mol), The functional monomer 3-mercaptopropionyloxyethyl methacrylate (0.6363g, 1.46mmol) and the dimer (0.6782g), the initiator BPO (1.3090g, 0.005mol) were mixed evenly, and added dropwise to temperature In the solvent xylene (7.6843g), propylene glycol methyl ether acetate (2.6903g) and heavy aromatics (2.6924g) at 135°C, the system was refluxed for 4 hours, and the mixture was added dropwise. The reaction was continued at a constant temperature for 3 hours, and the reaction was terminated to obtain a slightly yellowish transparent resin. The solid content of the resin was measured to be 69.32%, and the viscosity measured by the Grignard tube was 64.5s. Polymer is analyzed by three-detection gel permeation chromatography, and the results are as follows: light scattering weight average molecular weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com