Gemcitabine hydrochloride lyophilized powder for injection and preparation method thereof
A technology of gemcitabine hydrochloride and freeze-dried powder, which is applied in the direction of freeze-dried transportation, medical preparations containing no active ingredients, and medical preparations containing active ingredients. It can solve the problems of restricting large-scale production and achieve quality control. Small amount, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
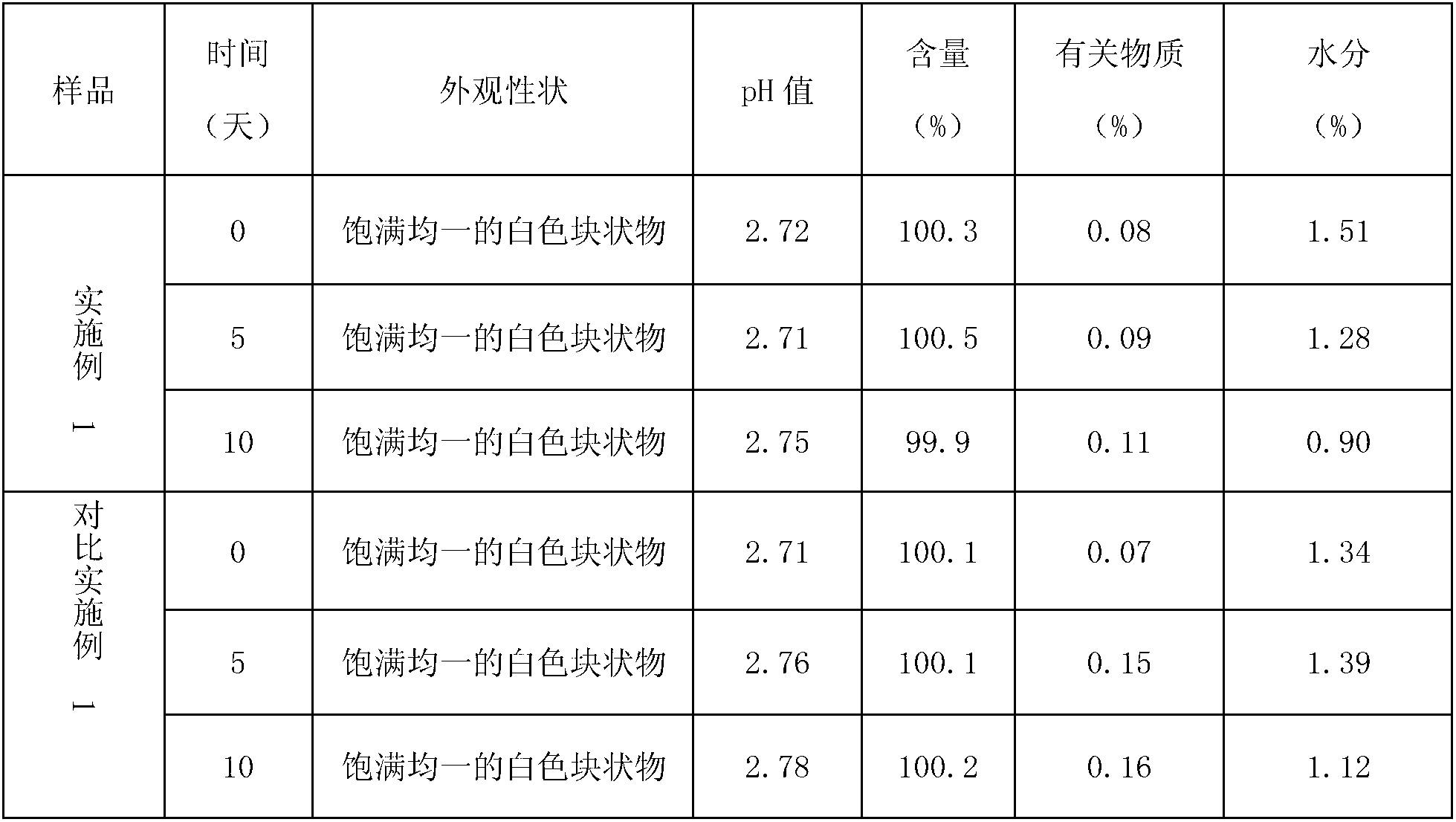
Examples
Embodiment 1
[0022] Prescription amount:
[0023] Gemcitabine Hydrochloride 228g
[0024] Lactose 410g
[0025] Sodium citrate 13.5g
[0026] Preparation Process:
[0027] Weigh the prescribed amount of lactose and sodium citrate, add 3.5L of water for injection to dissolve, then add the prescribed amount of gemcitabine hydrochloride, stir to dissolve, set the volume to 4L, heat to 50±2°C, add 0.05% of the prepared amount (g / ml) Activated carbon for needles, keep warm and adsorb for 30min, decarbonize, let cool, adjust pH=2.5~2.8 with 1mol / L hydrochloric acid solution or / and 1mol / L sodium hydroxide solution, filter and sterilize, fill the liquid medicine 1000 bottles, half-stoppered, samples into boxes, freeze-dried according to the following steps:
[0028] (1) Pre-freezing: adopt the slow freezing method, after the sample temperature drops to -35°C and the samples are all frozen and the color is consistent, continue to pre-freeze for 2 hours to completely freeze the samples;
[0029...
Embodiment 2
[0032] Prescription amount:
[0033] Gemcitabine Hydrochloride 228g
[0034] Lactose 365g
[0035] Sodium citrate 7.2g
[0036] Preparation process: with embodiment 1.
Embodiment 3
[0038] Prescription amount:
[0039] Gemcitabine Hydrochloride 1140g
[0040] Lactose 1930g
[0041] Sodium citrate 45g
[0042] Preparation Process:
[0043]Weigh the prescribed amount of lactose and sodium citrate, add 18L of water for injection to dissolve, then add the prescribed amount of gemcitabine hydrochloride, stir to dissolve, set the volume to 20L, heat to 50±2°C, add the prepared amount of 0.05% ( g / ml) activated carbon for needles, keep warm for 40min, decarbonize, let cool, adjust pH=2.5~3.0 with 1mol / L hydrochloric acid solution or / and 1mol / L sodium hydroxide solution, filter and sterilize, fill the liquid medicine, Half stoppered, the sample is put into the box, and freeze-dried according to the following steps:
[0044] (1) Pre-freezing: Use the slow freezing method. After the sample temperature drops to -35°C and the samples are all frozen and the color is consistent, continue to pre-freeze for 2 hours to completely freeze the samples;
[0045] (2) Subl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com