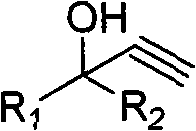
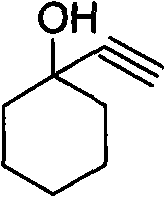
A method for purification and enhanced stability of alkynol compounds
A compound and stability technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve the problems of unqualified appearance, low added value, and low purity of alkynyl alcohol compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of 1-ethynyl-1-cyclohexanol
[0026] Add 150L tetrahydrofuran to the reaction kettle, add 22.4Kg of potassium tert-butoxide under stirring, cool to 0°C, pass acetylene gas, control the temperature below 5°C, and ventilate for 4 hours, add cyclohexanone 19.8Kg dropwise while passing acetylene gas , After the addition, the reaction was stirred for 2 hours, and the temperature was controlled below 0°C. The reaction solution was poured into ice water, the aqueous layer was extracted with dichloromethane, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and distilled under reduced pressure to collect fractions at 52-54°C / 2mm. 21.7Kg of light yellow liquid was obtained, the yield was 87.5%, and the purity was 95% (detected by GC method).
Embodiment 2
[0027] Example 2: Purification of 1-ethynyl-1-cyclohexanol
[0028] Crude product input and yield
[0029]
[0030] Add water to the reaction bottle, then add sodium bisulfite, and after it is completely dissolved, add crude 1-ethynyl-1-cyclohexanol to the reaction solution.
[0031] Heat and stir for 1 hour to stop the reaction. Static separation, the organic layer was collected, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, concentrated, and distilled to obtain a qualified product.
Embodiment 3
[0032] Example 3: Enhancement of the stability of 1-ethynyl-1-cyclohexanol
[0033] Qualified 1-ethynyl-1-cyclohexanol was examined under the following conditions respectively.
[0034]
[0035]
[0036] Results: The stability of 1-ethynyl-1-cyclohexanol can be greatly enhanced and the quality can be improved by adding polymerization inhibitor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com