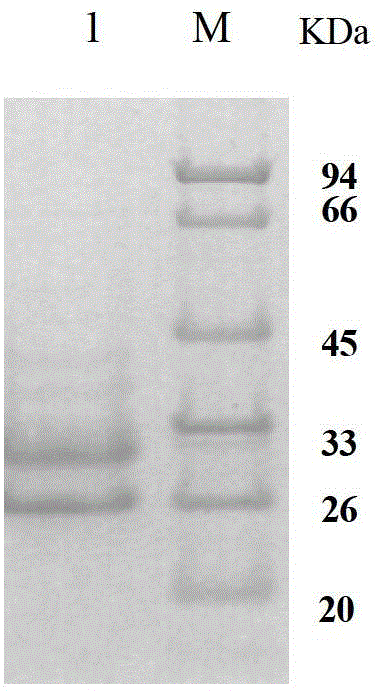
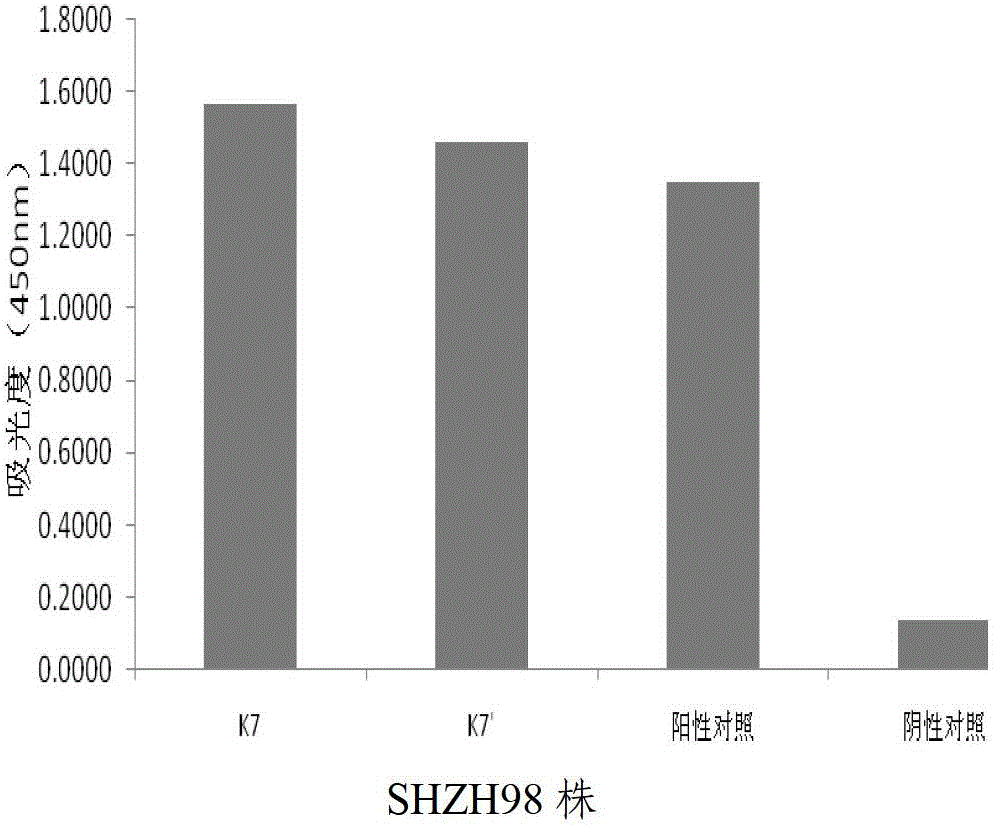
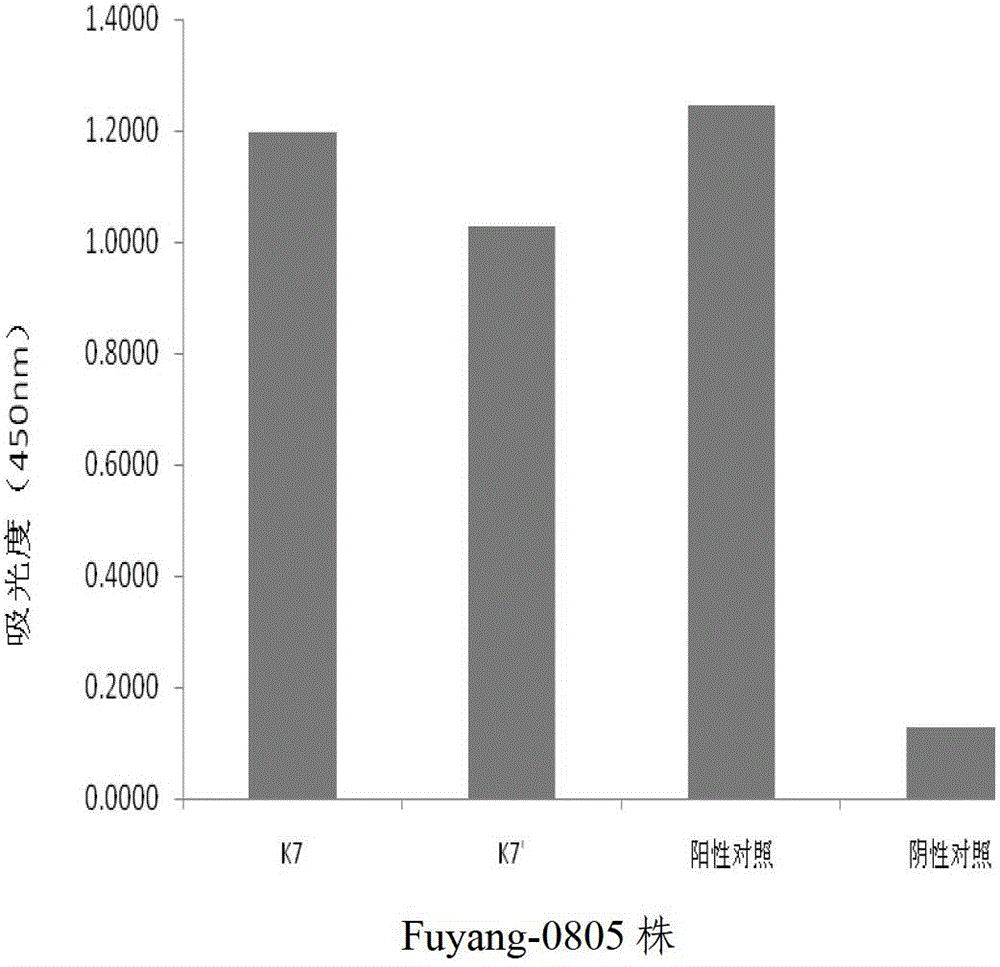
Human anti-EV71 (enterovirus 71) neutralizing antibody EV71FabK7 and preparation method and application thereof
A virus and antibody technology, applied in the direction of antiviral agents, antiviral immunoglobulin, botany equipment and methods, etc., can solve the problems of restriction, blood-borne disease infection, time-consuming, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction of Human Anti-EV71 Virus Antibody Library and Screening of Fab Antibody
[0033] 1.1 Materials and methods
[0034]1.1.1 Sources of viruses, cells and vectors: EV71 virus SHZH98 strain and FUYANG-0805 strain have been published in the literature (Yang F, Jin Q, He Y, Li L, Hou Y.2001. The complete genome of Enterovirus 71 China strain. Sci China C Life Sci and Wu Z, Yang F, Zhao R, Zhao L, Guo D, Jin Q.2009. Identification of small interfering RNAs which inhibit the replication of several Enterovirus 71 strains in China. J Virol Methods), by Provided by Institute of Pathogen Biology, Chinese Academy of Medical Sciences. The cells used for EV71 virus neutralization experiment in vitro were RD cells, which were purchased from ATCC. The bacterial strain is XL1-Blue (Stratagene, USA), and the vector pComb3H (provided by Scripps Research Institute, USA).
[0035] 1.1.2 Antigen preparation
[0036] Purification of EV71 virus: The culture supernatant...
Embodiment 2
[0109] Example 2 Application of Human Anti-EV71 Virus Neutralizing Antibody EV71FabK7
[0110] At present, there are no specific vaccines and drugs for the prevention and treatment of hand, foot and mouth disease. The immunoglobulins used clinically are all derived from the positive serum of human anti-EV71, which limits their large-scale preparation. Susceptible to infection with blood-borne diseases. The human source anti-EV71 virus genetically engineered antibody obtained by the invention is used to replace the blood source immunoglobulin, which provides a new approach for the treatment of hand, foot and mouth disease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com